9701_s04_ms
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS
GCE Advanced Subsidiary Level and GCE Advanced Level
MARK SCHEME for the June 2004 question papers
9701 CHEMISTRY
9701/01 Paper 1 (Multiple Choice), maximum raw mark 40
9701/02 Paper 2 (Theory 1 – Structured Questions),
maximum raw mark 60
9701/03 Paper 3 (Practical 1), maximum raw mark 25
9701/04 Paper 4 (Theory 2 – Structured Questions),
maximum raw mark 60
9701/05 Paper 5 (Practical 2), maximum raw mark 30
9701/06 Paper 6 (Options), maximum raw mark 40
These mark schemes are published as an aid to teachers and students, to indicate the requirements of the examination. They show the basis on which Examiners were initially instructed to award marks. They do not indicate the details of the discussions that took place at an Examiners’ meeting before marking began. Any substantial changes to the mark scheme that arose from these discussions will be recorded in the published Report on the Examination.
All Examiners are instructed that alternative co rrect answers and unexpected appro aches in candidates’ scripts must be given marks that fairly reflect the relevant kno wledge and skills demonstrated.
Mark schemes must be read in co njunctio n with the questio n papers and the Report on the Examination.
?CIE will not enter into discussion or correspondence in connection with these mark schemes.
CIE is publishing the mark schemes fo r the June 2004 questio n papers fo r mo st IGCSE and GCE Advanced Level syllabuses.
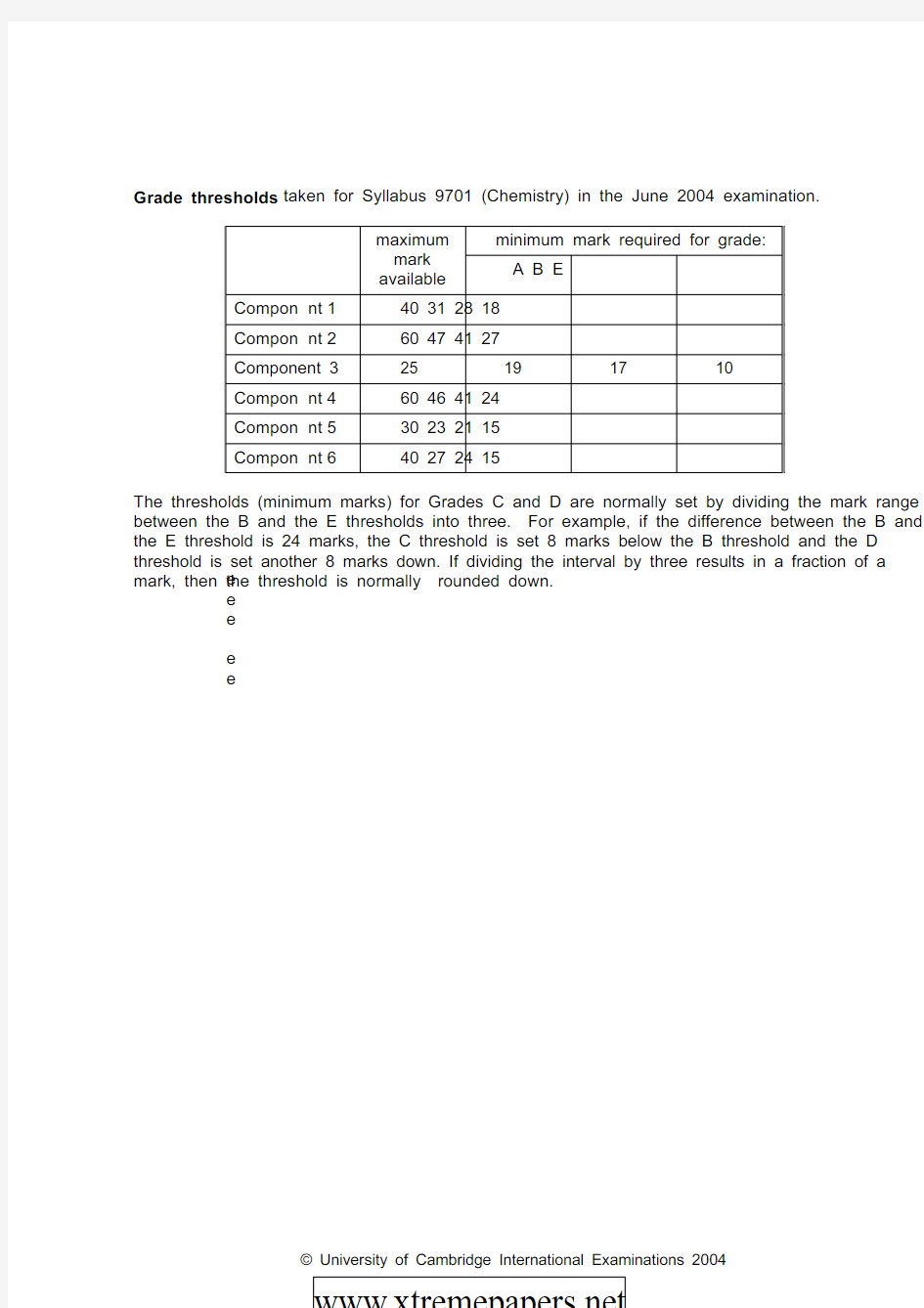
Grade thresholds taken for Syllabus 9701 (Chemistry) in the June 2004 examination.
minimum mark required for grade:
maximum
mark
available
A B E
Compon e nt
1
40 31 28 18
Compon e nt
2
60 47 41 27
Component 3 25 19 17 10
Compon e nt
4
60 46 41 24
Compon e nt
5
30 23 21 15
Compon e nt
6
40 27 24 15
The thresholds (minimum marks) for Grades C and D are normally set by dividing the mark range between the B and the E thresholds into three. For example, if the difference between the B and the E threshold is 24 marks, the C threshold is set 8 marks below the B threshold and the D threshold is set another 8 marks down. If dividing the interval by three results in a fraction of a mark, then the threshold is normally rounded down.
? University of Cambridge International Examinations 2004
JUNE 2004
GCE A AND AS LEVEL
MARK SCHEME
MAXIMUM MARK: 40
SYLLABUS/COMPONENT: 9701/01
CHEMISTRY
Paper 1 (Multiple Choice)
Page 1 Mark Scheme Syllabus Paper
CHEMISTRY – JUNE 2004 9701 1
Question Number Question
Number
Key
1 C 21 B
2 B 22 D
3 C 23 D
4 B 24 B
5 C 25 A
6 C 26 D
7 D 27 C
8 B 28 D
9 B 29 A
10 D 30 D
11 D 31 A
12 C 32 D
13 B 33 C
14 A 34 D
15 A 35 B
16 A 36 C
17 D 37 A
18 A 38 B
19 D 39 D
20 B 40 C
TOTAL 40
JUNE 2004
GCE A AND AS LEVEL
MARK SCHEME
MAXIMUM MARK: 60
SYLLABUS/COMPONENT: 9701/02
CHEMISTRY
Theory 1 (Structured Questions)
1 (a) The volume of the gas molecules / atoms / particles is insignificant compared
with the volume of the vessel.
There are no forces of attraction between the gas molecules.
All collisions by the gas molecules are perfectly elastics. Any two.
[2]
(b) (i) The pressure of / exerted by the gas. [1]
Pa / Nm -2 [1] (ii)The volume of the containing vessel [1] m3 / dm3 / cm3[1]
[1]
temperature
absolute
(iii) The
In K or
°C
[1]
+
273
(c) (i)pV ≈ w/m x RT
m = (0.103 x 8.31 x 297) / (99.5 x 103 x 63.8 x 10-6)
[1]
= 40.0[1]
argon
[1]
is
The
gas
(ii)The hydrogen bonds between ammonia molecules (1)
are stronger than the Van De Waals’ forces between N2 and Ar molecules (1)
Ammonia is polar / has a dipole (1)
(Any two) [2]
Total = [13]
2(a)1s22s2 2p63s2 3p3[1]
(b) 5 or V [1]
(c) (i)3NaOH + H3PO4 Na3PO4 + 3H2O [1]
(ii)(50 x 0.5) / 1000 = 0.025 (moles) [1]
(iii) conseq. on (i) 3 x .025 = 0.075 (moles) [1]
(d) (i) P4S3 + 8O2 P4O10 + 3SO2 balanced = 2 marks
2P2O5)
(or
OR + 6O2 P4O6 + 3SO2 unbalanced = 1 mark
2P2O3)
(or
[2]
(ii) P4O10 + 6H2O 4H3PO4[1]
OR P4O6 + 6H2O 4H3PO3
SO2 + H2O H2SO3[1]
(if
SO3 then
e.c.
f.) Total = [9]
3 (a) CO(NH 2)2 + H 2O 2NH 3 + CO 2 balanced equation (1) (1) colourless gas [2]
(b) (i) N 2 + 3H 2 ? 2NH 3
(ii) 100 ATMs or over 400 - 500°C iron catalyst (iii) Fertiliser, making nitric acid, explosives etc. 1 mark for each [4]
(c) (i) (1.2) / (2.4) = 1/20 or 0.05 moles [1] (ii) 2NH 3 + H 2SO 4 (NH 4)2SO 4 or equivalent [1] (iii) 0.025 mols of H 2SO 4 are required Vol. of 0.50 mol dm -3 H 2SO 4 required = (0.025 x 1000) / 0.5 = 50cm 3 [1]
(d)
1 mark for each diagram, 1 mark for each correct bond angle If not 3-dimensional diagram – 1 penalty.
[4]
(e) 4NH 3 + 3O 2 2N 2 + 6H 2O
[1]
N goes from -3 to 0 oxidation [1] O goes from 0 to -2 reduction [1]
Total = [16]
+
4 (a) (i) Acid or base, heating / reflux / warm [1] (ii) CH 3(CH 2)2CO 2CH 3 + H 2O CH 3(CH 2)2CO 2H + CH 3O H
[1] (iii) Solvents (polyesters not in AS syllabus, but allow as plastics, textiles) [1]
(b) (i)
1 mark for this diagram
(ii)
1 mark for ester link, 1 mark for rest of molecule [3] (c) (i) C 6H 12O 7
2 + 12 + 16 M r = 100 [1]
C H 2 = CH – CH = CH – CH 2CH 3 [1]
dehydration / elimination [1]
CH CH – O – C – CH 3
(d) A test for an alcohol (both alcohol and the product are alkenes) e.g. sodium bubbles of gas PC l 5 misty fumes H +/ Cr 2O 72- orange turned green conc. H 2SO 4 + carbonylic acid ester smell
NOT Br 2 or H +/MnO 4- as it tests positive for both 1 mark for specified test 1 mark for the relevant observation [2] Total = [11]
5 (a) Example (1) reason (1) MUST BE ORGANIC
e.g. PVC (1) used in food packaging (& needs to be inert) (1) Teflon / PTFE (1) used in non-stick kitchenware (1) Freons (1) used as deoderants, anaesthetics etc. (1) CC l 4 etc (1) solvent (1) 3 x 2 [6]
(b) (i) U.V. radiation [1] Breaks C-C l bond OR giving C l free radicals [1] These react with ozone [1]
(ii) e.g polypropene for PVC alkanes e.g. butane for aerosols
OR equivalent answers need not be organic e.g. N 2O as anaesthetic [2] Total = [11]
JUNE 2004
GCE A AND AS LEVEL
MARK SCHEME
MAXIMUM MARK: 25
SYLLABUS/COMPONENT: 9701/03
CHEMISTRY
Practical 1
Question 1
(a) Table 1.1
Give one mark if both weighings are to 2 dp or better, in the correct places in the table, and there is no error in subtraction.
Centres were instructed to provide between 1.70 g and 2.00 g of FA 1. If a
candidate’s mass is clearly a value in this range x10 or x 0.1 the mark for Table 1.1 will not be awarded but the “correct” value will be used in assessing the accuracy ratio .
[1]
1
(b) Titration Table 1.1
Give one mark if all final burette readings (except any labelled Rough) are to 2 dp and the readings are in the correct places in the table.
Do not give this mark if “impossible” burette readings (e.g. 23.47 cm 3) are given (initial or final readings).
Give one mark if there are two titres within 0.10 cm 3 and a “correct” average has been calculated.
See instructions in (f) and examples in (g) of Standing Instructions.
The subtraction of a Rough value need only be checked when the Rough value has been included in the selection for calculating the average.
Do not give this mark if there is an error in subtraction or there is no indication of the titres used to calculate the average (ticks/calculation). [2]
Accuracy
Check and correct if necessary the subtraction in Table 1.1 for the Supervisor. Use the rules in Standing Instructions to obtain a titration value for the Supervisor.
Calculate, correct to 2 decimal places, for each candidate:
necessary)
if (corrected Titre s Candidate' x necessary) if (corrected CO Na of mass s 'Candidate CO Na of mass s 'Supervisor 323
2 Compare the calculated value with the Titre value obtained by the Supervisor.
Assign accuracy marks as follows:
The spread penalty referred to in (g) may have to be applied using the table below
Accuracy marks
Spread Penalty
Mark
Difference from Supervisor
Range used / cm 3
De d
uction
6 up to 0.20 0.20+ to 0.25 1 5 0.20+ to 0.40 0.25+ to 0.30 2 4 0.40+ to 0.60 0.30+ to 0.40 3 3 0.60+ to 0.80 0.40+ to 0.50 4 2 0.80+ to 1.00 0.50+ to 0.70 5 1 1.00+ to 2.00 Greater than 0.70
6 0
Greater than 2.00
[6]
In all calculations, ignore evaluation errors if working is shown
(c) Give one mark for M r of Na 2CO 3 = 106
and one mark for
4
x CO Na for M calculated a CO Na of mass s 'candidate 3
2r 3
2
[2]
(d)
Give one mark for 1000
25
x (c) ans OR 10
1
x CO Na for M calculated a CO Na of mass s 'candidate 32r 32
[1]
(e) Give one mark for
2 x (d) to answer
1
[1]
(f) Give one mark for titre
s candidate'1000
x (e) to answer
and one further mark for a FULLY CORRECT answer to within 1% of the value calculated by the examiner using the titre/mass used by the candidate .
A candidate with an incorrect sub-section, who correctly starts a subsequent sub-section from first principles can gain the evaluation mark.
The correct value is given by: titre
s candidate'carbonate
sodium of mass s candidate' x 1.887
Do not award this evaluation mark if there are cancelling chemical errors or an M r other than 106 is used in the calculation .
Ignore rounding to less than 3 significant figures providing evaluation has been shown to 3 significant figures or better .
Examiners should calculate the value, correct to 3 significant figures, and record it in a ring close to the candidate’s value .
[2]
Total for Question 1 [15]
2FA 4 is a mixture of: FA 5 (Na2SO3 which is soluble in water) and
FA 6 (CaCO3 which is insoluble in water)
Tests
on
Filtrate
Test Observations
(a) To 1 cm depth of the filtrate in a test-tube, add 1
cm depth of aqueous barium chloride;
White precipitate
Accept white solution if a precipitate of
unspecified colour has also formed (no contrary
colours permitted)
followed by 2 cm depth of dilute hydrochloric acid Precipitate dissolves/disappears or
colourless/clear/transparent solution
Ignore any reference to slight white “haziness”
left in the solution or to evolution of gas.
Both parts of the observation are needed for the
one mark to be awarded.
[1]
(b) To 1 cm depth of the filtrate in a test-tube, add 1
cm depth of acidified aqueous potassium dichromate(VI). (Solution turns) green not blue/green
one mark
Do not penalise green precipitates[1]
(c) To 1 cm depth of the filtrate in a test-tube, add 2
cm depth of dilute hydrochloric acid.
Warm the solution and identify the gas given off.
Empty and wash away the contents of the tube at
the end of this test. (Gas with reducing properties)
A suitable test must be described:
turns chromate/dichromate green (blue/green is acceptable here)
or decolourises manganate(VII)
or
(Acidic gas)
turns a named indicator an appropriate colour
one mark
(Allow this mark on addition of HCl in test
(a) if not given in (c))
Ignore any lime-water test or reference to
CO2(some CaCO3 may pass through the filter paper)
[1]
(d) To 1 cm depth of the filtrate in a test-tube, add 2
cm depth of aqueous iodine. (Iodine) decolourised or colourless solution or
stated diminishing colour (eg. brown to yellow)
one mark [1]
one mark for identifying the anion as sulphite/SO32-
Give
providing two pieces of evidence are given in the conclusion that refer to
correct observations or there is equivalent unambiguous reference to tests in table.
the
i. white precipitate with barium chloride
ii dichromate(VI) turns green in test (b)
iii iodine decolourised
one from
iv acid/base indicator colour change in (c)
v dichromate(VI) turns green in (c)
[1]
Observation marks may be awarded in the supporting evidence section where
the candidate refers back to a specified test. (Beware of contrary statements)
Give one mark for stating that the anion behaves in (b) and (d) as a:
reductant / reducing agent / reducer / oxidisable species(providing a mark has been
given in (b) or (d)).
[1]
Tests on Residue
Test Observations
(e) Add 2 cm depth of hydrochloric acid to the residue
(FA 6) in the boiling-tube.
Use the solution formed in the following tests (f)
and (g).
(Gas evolved:)
A suitable test must be described:
turning lime water milky/cloudy/turbid/chalky
one mark [1]
(f) To 1 cm depth of the solution remaining after test
(e) add aqueous sodium hydroxide. White precipitate, insoluble in excess
Both parts of observation needed
one mark [1]
(g)
To 1 cm depth of the solution remaining after test
(e) add aqueous ammonia. No precipitate / no reaction /
solution remains colourless /
clear solution (remains but not formed)
One mark [1]
Observation marks may again be awarded in the supporting evidence section where the candidate refers back to a specified test. (Beware of contrary statements)
Give one mark if the cation and the anion match the results in tests (e), (f) and (g). and there is supporting evidence in the conclusion for each ion.
The anion is CO32-Allow carbonate from effervescence, fizzing or rapid evolution of gas
The cation is Ca2+
Matched observations:
Test (f) Test (g) Allowable deduction Unqualified White ppt
Do not allow if the ppt was
soluble in excess NaOH
No ppt Ca2+
White ppt insol in excess White ppt insol in excess Mg2+
White ppt insol in excess White ppt sol in excess No cation matches White ppt sol in excess White ppt insol in excess Al3+ or Pb2+
White ppt sol in excess White ppt sol in excess Zn2+ No ppt White ppt insol in excess No cation matches
No ppt White ppt sol in excess No cation matches
No ppt No ppt Ba2+
[1]
Total for Question 2 = [10]
Total for Paper = [25]
JUNE 2004
GCE A AND AS LEVEL
MARK SCHEME
MAXIMUM MARK: 60
SYLLABUS/COMPONENT: 9701/04
CHEMISTRY
Theory 2 (Structured Questions)
1 (a) Mg2+ + 2e-Mg [1]
(b) chlorine/C l2 [1]
(c) smaller A r[1]
radius/size
[1]
(atomic/ionic)
larger
solid
compound [1] (d) (i) the energy change when 1 mol of
ions [1] is formed from its gaseous
(ii) Mg2+ (g) + 2C l- (g) MgC l2 (s) charges + balancing [1]
state symbols[1]
(e) (i) LE (MgC l2) is greater than LE (NaC l) [1]
(because) Mg2+has higher charge / smaller radius than Na+ [1] (ii) LE (MgC l2) is greater than LE (CaC l2)
[1]
(because) Mg2+ is smaller than Ca2+ [1]
(f) LE = 349 – 122 – 494 – 107 – 411
= -785 (kJ mol-1) [3]
correct answer = [3], with – [1] for one error. OR mark as follows:
use of all 5 ?H values, with x1 multipliers [1]
correct signs for all ?H values [1]
answer
[1]
in
sign
negative
Total = [15]
2 (a) covalent (giant or macro) negates, as also does any reference to ionic bonding) [1]
(simple molecular is not enough – look for covalent)
[1]
tetrahedral
[1]
±1°)
(allow
(b) (i) plotting
138 – 151°C (stated in numbers, or read from the graph) [1]
(ii) (b. pt. increases due to) larger intermolecular / van der Waals / induced dipole
(NOT permanent dipole) / attractions [1]
due to the larger no. of electrons or more shells of electrons (in MX4) [1]
(c) (i) Si has empty low-lying orbitals or empty d-orbitals (C does not) [1]
(ii) SiC l4 + 2H2O SiO2 + 4HC l[1]
[or SiC l4 + 4H2O Si(OH)4 + 4HC l etc.]
(iii) (yes), because Ge also has empty (low lying d-) orbitals [1]
(d) (i) SiC l4 + 2Zn Si + 2ZnC l2 [NOT ionic equation] [1]
(ii) mass = 250 x 2 x 65.4/28.1
= 1164 (g) (actually 1163.7 – but allow 1160) [2]
allow e.c.f from the stoichiometry of the candidate’s equation e.g. allow 582g for
[2] marks if the equation shows the stoichiometry to be 1:1. But if 582g is obtained
because the candidate forgot to apply the stoichiometry as given in the equation,
award only [1] mark.
correct answer = [2], with – [1] for one error. OR marks as follows:
ration [1]
2:1
of
use
correct use of A r data for Si and Zn [1]
Total = [12]
?heterogeneous: different phases/states or homogeneous: same phase/state
?(heterogeneous): adsorption onto the surface
?the correct allocation of the terms heterogeneous and homogeneous to the two exemplar ?example of heterogeneous, e.g.Fe (in the Haber process)
e.g. N2 + 3H2 2NH3?equation,
?example of homogeneous, e.g. Fe3+ (in S2O82- + I-)
e.g. S2O82- + 2I- 2SO42- + I2 ?equation,
?how catalyst works, e.g. Fe3+ + I- Fe2+ + ?I2
[OR example: FeC l3(in Friedel-Crafts or chlorination etc. with CH3C l,C l2, Br2) equation, C6H6 +C l2 C6H5 C l + HC l
mode of action FeC l3 + C l2 FeC l4- + C l+ ]
Total = [8]
__________________________________________________________________________
[space for writing other examples using iron or its compounds you may come across. If in doubt consult your TL.
Mark as follows:
For heterogeneous: example [1] for homogeneous: example [1]
equation [1] equation [1]
[1]
action
of
mode candidates should include one example of each mode of catalysis]
