X-ray Absorption Spectroscopy (XAS)

X-ray Absorption Spectroscopy (XAS)
When the x-rays hit a sample, the oscillating electric field of the electromagnetic radiation interacts with the electrons bound in an atom. Either the radiation will be scattered by these electrons, or absorbed and excite the electrons.
x
A narrow parallel monochromatic x-ray beam of intensity I 0 passing through a sample of thickness x will get a reduced intensity I according to the expression: ln (I 0 /I) = μ x (1)
where μ is the linear absorption coefficient , which depends on the types of atoms and the density ρ of the material. At certain energies where the absorption increases drastically, and gives rise to an absorption edge . Each such edge occurs when the energy of the incident photons is just sufficient to cause excitation of a core electron of the absorbing atom to a continuum state, i.e . to produce a photoelectron. Thus, the energies of the absorbed radiation at these edges correspond to the binding energies of electrons in the K, L, M, etc, shells of the absorbing elements. The absorption edges are labelled in the order of increasing energy , K, L I , L II , L III , M I ,…., corresponding to the excitation of an electron from the 1s (2S ?), 2s (2S ?), 2p (2P ?), 2p (2
P 3/2), 3s (2S ?
), … orbitals (states), respectively. Bohr Atomic Model
ed K L L L Continuum
ge: 2 S ?
2
P ?2
P 32III
II I
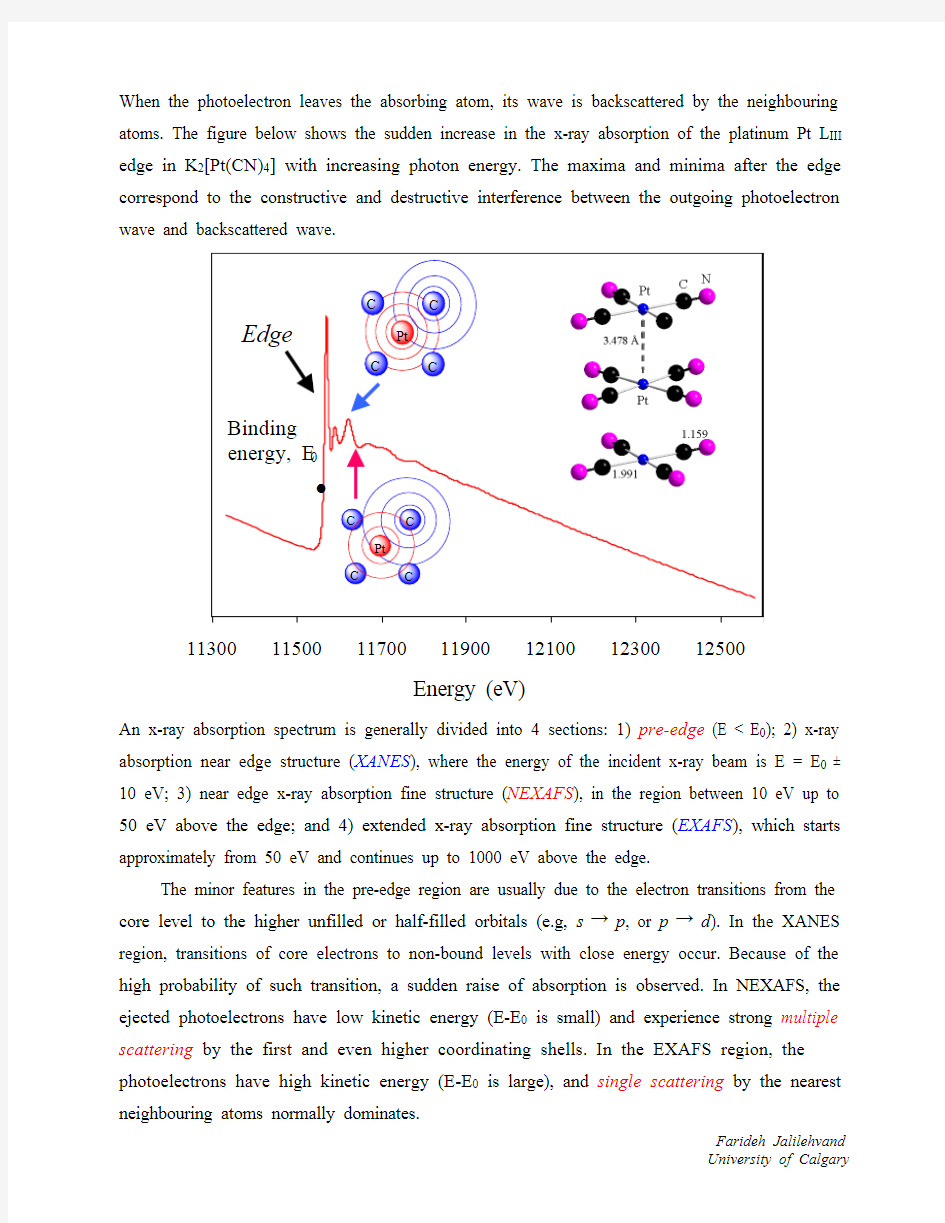
When the photoelectron leaves the absorbing atom, its wave is backscattered by the neighbouring atoms. The figure below shows the sudden increase in the x-ray absorption of the platinum Pt L III edge in K 2[Pt(CN)4] with increasing photon energy. The maxima and minima after the edge correspond to the constructive and destructive interference between the outgoing photoelectron wave and backscattered wave. 11300115001170011900121001230012500
Energy (eV)
μ (E )
An x-ray absorption spectrum is generally divided into 4 sections: 1) pre-edge (E < E 0); 2) x-ray absorption near edge structure (XANES ), where the energy of the incident x-ray beam is E = E 0 ± 10 eV; 3) near edge x-ray absorption fine structure (NEXAFS ), in the region between 10 eV up to 50 eV above the edge; and 4) extended x-ray absorption fine structure (EXAFS ), which starts approximately from 50 eV and continues up to 1000 eV above the edge.
The minor features in the pre-edge region are usually due to the electron transitions from the core level to the higher unfilled or half-filled orbitals (e.g, s → p , or p → d ). In the XANES region, transitions of core electrons to non-bound levels with close energy occur. Because of the high probability of such transition, a sudden raise of absorption is observed. In NEXAFS, the ejected photoelectrons have low kinetic energy (E-E 0 is small) and experience strong multiple scattering by the first and even higher coordinating shells. In the EXAFS region, the photoelectrons have high kinetic energy (E-E 0 is large), and single scattering by the nearest neighbouring atoms normally dominates.
11400.00.5
1.01.5
2.0011500
11600
11700
11800
11900
Multiple scattering
Single scattering
Home Research Publications Synchrotron XANES EXAFS XAS Measurement
(完整版)X射线光电子能谱分析(XPS)
第18章X射线光电子能谱分析 18.1 引言 固体表面分析业已发展为一种常用的仪器分析方法,特别是对于固体材料的分析和元素化学价态分析。目前常用的表面成分分析方法有:X射线光电子能谱(XPS), 俄歇电子能谱(AES),静态二次离子质谱(SIMS)和离子散射谱(ISS)。AES 分析主要应用于物理方面的固体材料科学的研究,而XPS的应用面则广泛得多,更适合于化学领域的研究。SIMS和ISS由于定量效果较差,在常规表面分析中的应用相对较少。但近年随着飞行时间质谱(TOF-SIMS)的发展,使得质谱在表面分析上的应用也逐渐增加。本章主要介绍X射线光电子能谱的实验方法。 X射线光电子能谱(XPS)也被称作化学分析用电子能谱(ESCA)。该方法是在六十年代由瑞典科学家Kai Siegbahn教授发展起来的。由于在光电子能谱的理论和技术上的重大贡献,1981年,Kai Siegbahn获得了诺贝尔物理奖。三十多年的来,X射线光电子能谱无论在理论上和实验技术上都已获得了长足的发展。XPS已从刚开始主要用来对化学元素的定性分析,业已发展为表面元素定性、半定量分析及元素化学价态分析的重要手段。XPS的研究领域也不再局限于传统的化学分析,而扩展到现代迅猛发展的材料学科。目前该分析方法在日常表面分析工作中的份额约50%,是一种最主要的表面分析工具。 在XPS谱仪技术发展方面也取得了巨大的进展。在X射线源上,已从原来的激发能固定的射线源发展到利用同步辐射获得X射线能量单色化并连续可调的激发源;传统的固定式X射线源也发展到电子束扫描金属靶所产生的可扫描式X射线源;X射线的束斑直径也实现了微型化,最小的束斑直径已能达到6μm大小, 使得XPS在微区分析上的应用得到了大幅度的加强。图像XPS技术的发展,大大促进了XPS在新材料研究上的应用。在谱仪的能量分析检测器方面,也从传统的单通道电子倍增器检测器发展到位置灵敏检测器和多通道检测器,使得检测灵敏度获得了大幅度的提高。计算机系统的广泛采用,使得采样速度和谱图的解析能力也有了很大的提高。 由于XPS具有很高的表面灵敏度,适合于有关涉及到表面元素定性和定量分析方面的应用,同样也可以应用于元素化学价态的研究。此外,配合离子束剥离技术和变角XPS技术,还可以进行薄膜材料的深度分析和界面分析。因此,XPS 方法可广泛应用于化学化工,材料,机械,电子材料等领域。 18.2 方法原理 X射线光电子能谱基于光电离作用,当一束光子辐照到样品表面时,光子可以被样品中某一元素的原子轨道上的电子所吸收,使得该电子脱离原子核的束缚,以一定的动能从原子内部发射出来,变成自由的光电子,而原子本身则变成一个激发态的离子。在光电离过程中,固体物质的结合能可以用下面的方程表示: E k = hν- E b - φs (18.1)
X射线光电子能谱仪
X射线光电子能谱分析 1 引言 固体表面分析业已发展为一种常用的仪器分析方法,特别是对于固体材料的分析和元素化学价态分析。目前常用的表面成分分析方法有:X射线光电子能谱(XPS), 俄歇电子能谱(AES),静态二次离子质谱(SIMS)和离子散射谱(ISS)。AES分析主要应用于物理方面的固体材料科学的研究,而XPS的应用面则广泛得多,更适合于化学领域的研究。SIMS和ISS由于定量效果较差,在常规表面分析中的应用相对较少。但近年随着飞行时间质谱(TOF-SIMS)的发展,使得质谱在表面分析上的应用也逐渐增加。本章主要介绍X射线光电子能谱的实验方法。 X射线光电子能谱(XPS)也被称作化学分析用电子能谱(ESCA)。该方法是在六十年代由瑞典科学家Kai Siegbahn教授发展起来的。由于在光电子能谱的理论和技术上的重大贡献,1981年,Kai Siegbahn获得了诺贝尔物理奖。三十多年的来,X射线光电子能谱无论在理论上和实验技术上都已获得了长足的发展。XPS已从刚开始主要用来对化学元素的定性分析,业已发展为表面元素定性、半定量分析及元素化学价态分析的重要手段。XPS的研究领域也不再局限于传统的化学分析,而扩展到现代迅猛发展的材料学科。目前该分析方法在日常表面分析工作中的份额约50%,是一种最主要的表面分析工具。 在XPS谱仪技术发展方面也取得了巨大的进展。在X射线源上,已从原来的激发能固定的射线源发展到利用同步辐射获得X射线能量单色化并连续可调的激发源;传统的固定式X射线源也发展到电子束扫描金属靶所产生的可扫描式X射线源;X射线的束斑直径也实现了微型化,最小的束斑直径已能达到6 m 大小, 使得XPS在微区分析上的应用得到了大幅度的加强。图像XPS技术的发展,大大促进了XPS在新材料研究上的应用。在谱仪的能量分析检测器方面,也从传统的单通道电子倍增器检测器发展到位置灵敏检测器和多通道检测器,使得检测灵敏度获得了大幅度的提高。计算机系统的广泛采用,使得采样速度和谱图的解析能力也有了很大的提高。 由于XPS具有很高的表面灵敏度,适合于有关涉及到表面元素定性和定量分析方面的应用,同样也可以应用于元素化学价态的研究。此外,配合离子束剥离技术和变角XPS技术,还可以进行薄膜材料的深度分析和界面分析。因此,XPS方法可广泛应用于化学化工,材料,机械,电子材料等领域。 2 方法原理 X射线光电子能谱基于光电离作用,当一束光子辐照到样品表面时,光子可以被样品中某一元素的原子轨道上的电子所吸收,使得该电子脱离原子核的束缚,以一定的动能从原子内部发射出来,变成自由的光电子,而原子本身则变成
光电子能谱分析法基本原理
第十四章 X-射线光电子能谱法 14.1 引言 X-射线光电子谱仪(X-ray Photoelectron Spectroscopy,简称为XPS),经常又被称为化学分析用电子谱(Electron Spectroscopy for Chemical Analysis,简称为ESCA),是一种最主要的表面分析工具。自19世纪60年代第一台商品化的仪器开始,已经成为许多材料实验室的必不可少的成熟的表征工具。XPS发展到今天,除了常规XPS外,还出现了包含有Mono XPS (Monochromated XPS, 单色化XPS,X射线源已从原来的激发能固定的射线源发展到利用同步辐射获得X射线能量单色化并连续可调的激发源), SAXPS ( Small Area XPS or Selected Area XPS, 小面积或选区XPS,X射线的束斑直径微型化到6μm) 和iXPS(imaging XPS, 成像XPS)的现代XPS。目前,世界首台能量分辨率优于1毫电子伏特的超高分辨光电子能谱仪(通常能量分辨率低于1毫电子伏特)在中日科学家的共同努力下已经研制成功,可以观察到化合物的超导电子态。现代XPS拓展了XPS的内容和应用。 XPS是当代谱学领域中最活跃的分支之一,它除了可以根据测得的电子结合能确定样品的化学成份外,XPS最重要的应用在于确定元素的化合状态。XPS可以分析导体、半导体甚至绝缘体表面的价态,这也是XPS的一大特色,是区别于其它表面分析方法的主要特点。此外,配合离子束剥离技术和变角XPS技术,还可以进行薄膜材料的深度分析和界面分析。XPS表面分析的优点和特点可以总结如下: ⑴固体样品用量小,不需要进行样品前处理,从而避免引入或丢失元素所造成的错误分析 ⑵表面灵敏度高,一般信息采样深度小于10nm ⑶分析速度快,可多元素同时测定 ⑷可以给出原子序数3-92的元素信息,以获得元素成分分析 ⑸可以给出元素化学态信息,进而可以分析出元素的化学态或官能团 ⑹样品不受导体、半导体、绝缘体的限制等 ⑺是非破坏性分析方法。结合离子溅射,可作深度剖析 目前,XPS主要用于金属、无机材料、催化剂、聚合物、涂层材料、纳米材料、矿石等各种材料的研究,以及腐蚀、摩擦、润滑、粘接、催化、包覆、氧化等过程的研究,也可以用于机械零件及电子元器件的失效分析,材料表面污染物分析等。 14.2 基本原理 XPS方法的理论基础是爱因斯坦光电定律。用一束具有一定能量的X射线照射固体样品,入射光子与样品相互作用,光子被吸收而将其能量转移给原子的某一壳层上被束缚的电子,此时电子把所得能量的一部分用来克服结合能和功函数,余下的能量作为它的动能而发射出来,成为光电子,这个过程就是光电效应。 该过程可用下式表示: hγ=E k+E b+E r(14.1) 式中: hγ:X光子的能量(h为普朗克常数,γ为光的频率);
γ射线能谱的测量
(一) γ射线能谱的测量 摘要: 本实验将了解闪烁探测器谱仪的工作原理及其使用;学习分析实验测量的137Cs 和60Co γ谱之谱形和γ射线能谱的刻度测定谱仪的能量分辨率,本实验的目的是了解NaI(Tl)闪烁谱仪的原理、特性与结构,掌握NaI(Tl)闪烁谱仪的使用方法和γ射线能谱的刻度。 关键词:γ 射线 Na(Tl)闪烁探测器 能谱图 单道脉冲幅度分析器 引言: 闪烁探测器是利用某些物质在射线作用下会发光的特性来探测射线的仪器。它的主要优点是:既能探测各种带电粒子,又能探测中性粒子;既能测量粒子强度,又能测量粒子能量;且探测效率高,分辨时间短。它在核物理研究和放射性同位素测量中得到广泛的应用。核物理的发展,不断地为核能装置的设计提供日益精确的数据,新的核技术,如核磁共振、穆斯堡尔谱学、晶体的沟道效应和阻塞效应,以及扰动角关联技术等都迅速得到应用。核技术的广泛应用已成为现代化科学技术的标志之 正 文: 实验原理 1.闪烁谱仪结构与工作原理 NaI(Tl)闪烁谱仪结构如图。整个仪器由探头(包括闪烁体、光电倍增管、射极跟随器),高压电源,线性放大器、多道脉冲幅度分析器几部分组成。射线通过闪烁体时,闪烁体的发光强度与射线在闪烁体内损失的能量成正比。带电粒子(如α、β粒子)通过闪烁体时,将引起大量的分子或原子的激发和电离,这些受激的分子或原子由激发态回到基态时就放出光子;不带电的γ射线先在闪烁体内产生光电子、康普顿电子及正、负电子对(当Eγ>1.02MeV时),然后这些电子使闪烁体内的分子或原子激发和电离而发光。闪烁体发出的光子被闪烁体外的光反射层反射,会聚到光电倍增管的光电阴极上,打出光电子。光阴极上打出的光电子在光电倍增管中倍增出大量电子,最后为阳极吸收形成电压脉冲。每产生一个电压脉冲就表示有一个粒子进入探测器。由于电压脉冲幅度与粒子在闪烁体内消耗的能量(产生的光强)成正比,所以根据脉冲幅度的大小可以确定入射粒子的能量。利用脉冲幅度分析器可以测定入射射线的能谱。 由原子物理学中可知γ射线与物质的相互作用主要是光电效应、康普顿效应和正、负电子对产生这三种过程分别如下: (1)光电效应。入射γ粒子把能量全部转移给原子中的束缚电子,而把束缚电子打出来形成光电子。由于束缚电子的电离能E1一般远小于入射γ射线能量Eγ,所以光电子的动能近似等于入射γ射线的能量E光电=Eγ-E1≈Eγ (2)康普顿效应。核外电子与入射γ射线发生康普顿散射,设入射γ光子能量为h,散射
时间计算题汇总
地理时间计算部分专题练习 1、9月10日在全球所占的围共跨经度90°,则时间为:( ) A. 10日2时 B. 11日2时 C. 10日12时 D. 11日12时 3、时间为2008年3月1日的2点,此时与处于同一日期的地区围约占全球的:( ) A. 一半 B. 三分之一 C. 四分之一 D. 五分之一 4、图中两条虚线,一条是晨昏线,另一条两侧大部分地区日期不同,此时地球公转速度较慢。若图中的时间为7日和8日,甲地为( ) A.7日4时 B.8日8时 C.7月8时 D.8月4时 2004年3月22日到4月3日期间,可以看到多年一遇的“五星连珠”天象奇观。其中水星是最难一见的行星,观察者每天只有在日落之后的1 小时才能看到它。图中阴影部分表示黑夜,中心点为极地。回答5—7题 5.图4中①②③④四地,可能看到“五星连珠”现象的是( ) A .① B .② C .③ D .④ 6.在新疆的吐鲁番(约890 E )观看五星连珠现象,应该选择的时间段(时间)是( ) A .18时10分至19时 B .16时10分至17时 C .20时10分至21时 D.21时10分至22时 7.五星连珠中,除了水星外,另外四颗星是( ) A .金星、木星、土星、天狼星 B .金星、火星、木星、海王星 C .火星、木星、土星、天王星 D .金星、火星、土星、木星 (2002年)下表所列的是12月22日甲、乙、丙、丁四地的白昼时间,根据表中数据回答下8-10题。 甲地 乙地 丙地 丁地 白昼长 5∶30 9∶09 11∶25 13∶56 8、四地中属于南半球的是( ) A.甲地 B.乙地 C.丙地 D.丁地 9、四地所处纬度从高到低顺序排列的是( ) A.甲乙丙丁 B.甲乙丁丙 C.丙丁乙甲 D.丁丙乙甲 10、造成四起白昼时间差异的主要因素是( ) ①地球的公转 ②地球自转 ③黄赤交角的存在 ④地方时的不同 A.①② B.②③ C.③④ D.①③ 2002年1月1日,作为欧洲联盟统一货币的欧元正式流通,这将对世界金融的整体格局产生重要影响。回 答4-5题: 11、假定世界各金融市场均在当地时间上午9时开市,下午5时闭市。如果某投资者上午9时在法兰克福(东经8.50 )市场买进欧元,12小时后欧元上涨,投资者想尽快卖出欧元,选择的金融市场应位于:( ) A.东京(东经139.50 ) B.(东经1140 ) C.伦敦 D.纽约(西经740 ) ① ④ ② ③
X射线光电子能谱模板
第二十三章 X射线光电子能谱 1954年以瑞典Siegbahn教授为首的研究小组观测光峰现象,不久又发现了原子内层电子能级的化学位移效应,于是提出了ESCA(化学分析电子光谱学)这一概念。由于这种方法使用了铝、镁靶材发射的软X射线,故也称为X-光电子能谱(X-ray Photoelectron Spectroscopy)。X光电子能谱分析技术已成为表面分析中的常规分析技术,目前在催化化学、新材料研制、微电子、陶瓷材料等方面得到了广泛的应用。 23.1 基本原理 固体表面分析,特别是对固体材料的分析和元素化学价态分析,已发展为一种常用的仪器分析方法。目前常用的表面成分分析方法有:X射线光电子能谱(XPS), 俄歇电子能谱(AES),静态二次离子质谱(SIMS)和离子散射谱(ISS)。AES分析主要应用于物理方面的固体材料(导电材料)的研究,而XPS的应用面则广泛得多,更适合于化学领域的研究。SIMS 和ISS由于定量效果较差,在常规表面分析中的应用相对较少。但近年随着飞行时间二次离子质谱(TOF-SIMS)的发展,使得质谱在表面分析上的应用也逐渐增加。 X射线光电子能谱最初是由瑞典科学家K.Siegbahn等经过约20年的努力而建立起来的,因在化学领域的广泛应用,被称为化学分析用电子能谱(ESCA)。由于最初的光源采用了铝、镁等的特性软X射线,该技术又称为X射线光电子能谱(XPS)。1962年,英国科学家D.W.Turner等建造出以真空紫外光作为光源的光电子能谱仪,在分析分子内价电子的状态方面获得了巨大成功,同时又用于固体价带的研究,与X射线光电子能谱相对照,该方法称为紫外光电子能谱(UPS) XPS的原理是基于光的电离作用。当一束光子辐射到样品表面时,样品中某一元素的原子轨道上的电子吸收了光子的能量,使得该电子脱离原子的束缚,以一定的动能从原子内部发射出来,成为自由电子,而原子本身则变成处于激发态的离子,如图23-1所示。在光电离过程中,固体物质的结合能可用下面的方程式表示: E b=hγ- E k -φs(23-1) 式中: E k为射出的光子的动能;hγ为X射线源的能量;E b为特定原子轨道上电子的电离能或结合能(电子的结合能是指原子中某个轨道上的电子跃迁到表面Fermi能级(费米能级)所需要的能量);φs为谱仪的功函数。 由于φs是由谱仪的材料和状态决定,对同一台谱仪来说是一个常数,与样品无关,其平均值为3 eV ~4eV。因此,(1)式可简化为: E b =hγ- E k’ (23-2) 由于E k’可以用能谱仪的能量分析器检出,根据式(23-2)就可以知道E b。在XPS分析中,由于X射线源的能量较高,不仅能激发出原子轨道中的价电子,还可以激发出内层轨道电子,所射出光子的能量仅与入射光子的能量及原子轨道有关。因此,对于特定的单色激发光源及特定的原子轨道,其光电子的能量是特征性的。当固定激发光源能量时,其光子的能量仅与元素的种类和所电离激发的原子轨道有关,对于同一种元素的原子,不同轨道上的电子的结合能不同。所以可用光电子的结合能来确定元素种类。图23-1表示固体材料表面受X射线激发后的光电离过程[1]。
X射线光电子能谱(XPS)谱图分析
一、X光电子能谱分析的基本原理 X光电子能谱分析的基本原理:一定能量的X光照射到样品表面,和待测物质 发生作用,可以使待测物质原子中的电子脱离原子成为自由电子。该过程可用 下式表示: hn=Ek+Eb+Er (1) 其中:hn:X光子的能量;Ek:光电子的能量;Eb:电子的结合能;Er:原子的 反冲能量。其中Er很小,可以忽略。 对于固体样品,计算结合能的参考点不是选真空中的静止电子,而是选用费米 能级,由内层电子跃迁到费米能级消耗的能量为结合能Eb,由费米能级进入真 空成为自由电子所需的能量为功函数Φ,剩余的能量成为自由电子的动能Ek,式(1)又可表示为: hn=Ek+Eb+Φ(2) Eb=hn-Ek-Φ(3)仪器材料的功函数Φ是一个定值,约为 4 eV,入射X光子能量已知,这样, 如果测出电子的动能Ek,便可得到固体样品电子的结合能。各种原子,分子的 轨道电子结合能是一定的。因此,通过对样品产生的光子能量的测定,就可以 了解样品中元素的组成。元素所处的化学环境不同,其结合能会有微小的差别,这种由化学环境不同引起的结合能的微小差别叫化学位移,由化学位移的大小 可以确定元素所处的状态。例如某元素失去电子成为离子后,其结合能会增加,如果得到电子成为负离子,则结合能会降低。因此,利用化学位移值可以分析 元素的化合价和存在形式。 二、电子能谱法的特点 (1)可以分析除H和He以外的所有元素;可以直接测定来自样品单个能级光电 发射电子的能量分布,且直接得到电子能级结构的信息。(2)从能量范围看,如果把红外光谱提供的信息称之为“分子指纹”,那么电子能谱提供的信息可称 作“原子指纹”。它提供有关化学键方面的信息,即直接测量价层电子及内层 电子轨道能级。而相邻元素的同种能级的谱线相隔较远,相互干扰少,元素定 性的标识性强。 (3)是一种无损分析。 (4)是一种高灵敏超微量表面分析技术,分析所需试样约10-8g即可,绝对灵敏
案例解析X射线光电子能谱(XPS)八大应用!
【干货】玩转XPS丨案例解析X射线光电子能谱(XPS)八大应用! 表面分析技术 (Surface Analysis)是对材料外层(the Outer-Most Layers of Materials (<100nm))的研究的技术。 X射线光电子能谱简单介绍 XPS是由瑞典Uppsala大学的K. Siegbahn及其同事历经近20年的潜心研究于60年代中期研制开发出的一种新型表面分析仪器和方法。鉴于K. Siegbahn教授对发展XPS领域做出的重大贡献,他被授予1981年诺贝尔物理学奖。 X射线激发光电子的原理 XPS现象基于爱因斯坦于1905年揭示的光电效应,爱因斯坦由于这方面的工作被授予1921年诺贝尔物理学奖; X射线是由德国物理学家伦琴(Wilhelm Conrad R?ntgen,l845-1923)于1895年发现的,他由此获得了1901年首届诺贝尔物理学奖。
X射线光电子能谱(XPS ,全称为X-ray Photoelectron Spectroscopy)是一种基于光电效应的电子能谱,它是利用X射线光子激发出物质表面原子的内层电子,通过对这些电子进行能量分析而获得的一种能谱。 这种能谱最初是被用来进行化学分析,因此它还有一个名称,即化学分析电子能谱(ESCA,全称为Electron Spectroscopy for Chemical Analysis)。XPS谱图分析中原子能级表示方法 XPS谱图分析中原子能级的表示用两个数字和一个小字母表示。例如:3d5/2(1)第一个数字3代表主量子数(n); (2)小写字母代表角量子数; (3)右下角的分数代表内量子数j
X射线能谱分析
X射线能谱分析简介 导言: 早在二十世纪年代中期就开始了X射线能谱分析课题的研究。例如,Parrish和Kohler(1956)曾指出用分解正比计数器脉冲高度谱的方法进行X射线能量分析的可能性。后来Dolby(1959、1960)发展了这种方法并且获得了Be、C、O等超轻元素的扫描X射线图像。同年,Duncumb提出一种用纯元素的标准谱拟合实际谱进行分析的方法。而Birks等人用正比计数器和一台400道多道分析器配合,在电子探针中首次进行了能谱分析。到了1968年,Fitzgerald、Keil和Heinrich等人开始把锂漂移硅探测器用到了电子探针中。 由于锂漂移硅探测器有一些独到的优点,得到了有关专家的广泛重视。在1963年和1970年,美国材料试验学会先后两次就能谱分析技术进行了专门的讨论,促进了能谱技术的发展。例如,在1966年,锂漂移硅探测器的能量分辨率还只能达到约800eV,但是到了1970年,就迅速提高到约150eV。探测器分辨率的提高,反过来促进了能谱分析方法及其相关技术的迅速发展。 目前,能谱分析系统已成为电子探针和扫描电镜/透射电镜微区分析的一项标准设备,同时与其相关的波谱分析、电子被散射衍射等有机结合,愈来愈成为微区分析中不可或缺的分析手段。 锂漂移硅探测器简述: 能谱分析系统的心脏是一只硅晶体二极管,它是由一块p型硅晶片经锂(向硅中)扩散和飘移后制成的,因此称为锂漂移硅探测器
(Lithium Drifted Silicon Detector),通常缩写为Si(Li)探测器。 我们知道硅是一种典型的半导体材料。硅晶体的结晶结构与金刚石结构相同,即为面心立方体结构,每个晶胞含有两个硅原子,每个硅原子有四个价电子(两个3s电子,两个3p电子)。在晶体中,每个原子与相邻四个原子构成四条共价键。根据能带理论,四个价电子形成四个能带,由于每个格点上有8个价电子,因此,如果格点数为N,则四个能带上将填满8N个电子,这种能带称为满带。满带的上方有一个能隙,称为禁带,禁带中不可能有任何电子,或者说,不可能存在其能量相当于禁带能量的电子。在禁带上面有很多可能的能带-----导带。在纯净而完整的晶体中,导带中没有电子,因此呈绝缘体特性。但是,即使纯度非常高的硅单晶,仍有极少量的杂质存在,而且难免有些晶格缺陷,加上硅的禁带宽度较小(~1.1eV),在热骚动下可能有极少量的电子进入导带,因此硅晶体有一定程度的导电性。温度愈高,由于热骚动而进入导带的电子愈多,晶体的导电性就愈强,因而使硅晶体成为一种典型的半导体。 半导体的导电率取决于杂质的类型和含量。杂质的作用是这样的:假如有一种五价杂质(P,As等)参入硅中,它将取代硅原子的位置,用四个价电子与相邻的四个硅原子结合而维持原来的四条共价键,并把多余的一个电子释放出去,被释放的电子很可能进入导带,使晶体呈电子性导电,这种晶体就称为n型半导体。如果掺入的杂质是三价原子,那么这些杂质将会俘获满带中的电子而使满带中出现空穴,从而使晶体成为p型半导体。在硅中常见的一种杂质是硼(B),它的
小学三年级数学《简单的时间计算》教案范文三篇
小学三年级数学《简单的时间计算》教案范文三篇时间计算是继二十四时计时法的学习之后安排的一个内容。下面就是小编给大家带来的小学三年级数学《简单的时间计算》教案范文,欢迎大家阅读! 教学目标: 1、利用已学的24时记时法和生活中对经过时间的感受,探索简单的时间计算方法。 2、在运用不同方法计算时间的过程中,体会简单的时间计算在生活中的应用,建立时间观念,养成珍惜时间的好习惯。 3、进一步培养课外阅读的兴趣和多渠收集信息的能力。 教学重点: 计算经过时间的思路与方法。 教学难点: 计算从几时几十分到几时几十分经过了多少分钟的问题。 教学过程: 一、创设情景,激趣导入 1、谈话:小朋友你们喜欢过星期天吗?老师相信我们的星期天都过得很快乐!明明也有一个愉快的星期天,让我们一起来看看明明的一天,好吗? 2、小黑板出示明明星期天的时间安排。 7:10-7:30 起床、刷牙、洗脸; 7:40-8:20 早锻炼; 8:30-9:00 吃早饭; 9:00-11:00 看书、做作业 …… 3、看了刚才明明星期天的时间安排,你知道了什么?你是怎么知道的?你还想知道什么?
二、自主探究,寻找方法 1、谈话:小明在星期天做了不少的事,那你知道小明做每件事情用了多少时间吗?每 个小组从中选出2件事情计算一下各用了多少时间。 (1)分组学习。 (2) 集体交流。 2、根据学生的提问顺序学习时间的计算。从整时到整时经过时间的计算。 (1)学生尝试练习9:00-11:00明明看书、做作业所用的时间。 (2)交流计算方法:11时-9时=2小时。 3、经过时间是几十分钟的时间计算。 (1)明明从7:40到8:20进行早锻炼用了多少时间呢? 出示线段图。 师:7:00-8:00、8:00-9:00中间各分6格,每格表示10分钟,两个线段下边 的箭头分别指早锻炼开始的时间和结束的时间,线段图涂色部分表示早锻炼的时间。谈话:从图上看一看,从7时40分到8时经过了多少分钟?(20分)从8时到8时20分又经过 了多少时间?所以一共经过了多少分钟。(20+20=40分)小朋友们,如果你每天都坚持锻炼 几十分钟,那你的身体一定会棒棒的。 (2)你还能用别的方法计算出明明早锻炼的时间吗?(7:40-8:40用了一个小时,去掉 多算的20分,就是40分。或者7:20-8:20用了1个小时,去掉多算的20分,就是 40分。) (3)练习:找出明明的一天中做哪些事情也用了几十分钟? 你能用自己喜欢的方法计算出明明做这几件事情用了几十分钟吗?你是怎么算的? 三、综合练习,巩固深化 1、想想做做1:图书室的借书时间。你知道图书室每天的借书时间有多长吗? 学生计算。 (1)学生尝试练习,交流计算方法。 (2)教师板书。 2、想想做做2。 (1)学生独立完成。 (2)全班交流。
(完整word版)X射线能谱仪工作原理及谱图解析1X射线能谱仪分析原理X射线能谱
X射线能谱仪工作原理及谱图解析 1、X射线能谱仪分析原理 X射线能谱仪作为扫描电镜的一个重要附件,可被看成是扫描电镜X射线 信号检测器。其主要对扫描电镜的微区成分进行定性、定量分析,可以分析元素周期表中从B-U的所有元素信息。其原理为:扫描电镜电子枪发出的高能电子进入样品后,受到样品原子的非弹性散射,将能量传递给该原子。该原子内壳层的电子被电离并脱离,内壳层上出现一个空位,原子处于不稳定的高能激发态。在激发后的10-12s内原子便恢复到最低能量的基态。在这个过程中,一系列外层 电子向内壳层的空位跃迁,同时产生X射线,释放出多余的能量。对任一原子而言,各个能级之间的能量差都是确定的,因此各种原子受激发而产生的X射线的能量也都是确定的(图1)。 X射线能谱仪收集X射线,并根据其能量对其记数、分类,从而对元素进 行定性、定量分析。 图1. 粒子间相互作用产生特征X射线 本所能谱仪型号为:BRUKER X-Flash 5010,有四种检测模式:点扫描,区域扫描,线扫描,面扫描。 2、能谱仪检测模式介绍及参数解读 2.1 点扫描及区域扫描模式
图2 X射线能谱仪点扫描(A)、选区扫描(B)报告 点扫描与选区扫描主要用于对元素进行定性和定量分析,确定选定的点或区域范围内存在的所有元素种类,并对各种元素的相对含量进行计算。能谱检测对倍数要求不高,不同倍数条件下检测结果差异不大,关键在于选取检测的部位。一般选择较大的块体在5000倍以下检测,因为X射线出射深度较深,除金属或陶瓷等非常致密的材料外,一般的块体在20kV加速电压下,X射线出射深度2μm左右,且点扫描的范围也在直径2μm左右。因此块体太小或倍数过大,都会造成背景严重,测量准确度下降。 此外,最好选择比较平整的区域检测,因为电子打在坑坑洼洼的样品表面,X射线出射深度差别较大,定量信息不够准确。特别低洼的区域,几乎检测不到信号,或信号很弱,得到的结果也便不准确。 第三,电子束与轻元素相会作用区域较大,干扰更强,因此轻元素的定量比重元素更加不准确。如C、N等元素,定量结果可能偏差较大。 点扫描与区域扫描测试报告相似,均由三部分组成,一张样品表面形貌照片,
古代时间的计算方法
中国古代时间的计算方法(1) 现时每昼夜为二十四小时,在古时则为十二个时辰。当年西方机械钟表传入中国,人们将中西时点,分别称为“大时”和“小时”。随着钟表的普及,人们将“大时”忘淡,而“小时”沿用至今。 古时的时(大时)不以一二三四来算,而用子丑寅卯作标,又分别用鼠牛虎兔等动物作代,以为易记。具体划分如下:子(鼠)时是十一到一点,以十二点为正点;丑(牛)时是一点到三点,以两点为正点;寅(虎)时是三点到五点,以四点为正点;卯(兔)时是五点到七点,以六点为正点;辰(龙)时是七点到九点,以八点为正点;巳(蛇)时是九点到十一点,以十点为正点;午(马)时是十一点到一点,以十二点为正点;未(羊)时是一点到三点,以两点为正点;申(猴)时是三点到五点,以四点为正点;酉(鸡)时是五点到七点,以六点为正点;戌(狗)时是七点到九点,以八点为正点;亥(猪)时是九点到十一点,以十点为正点。 古人说时间,白天与黑夜各不相同,白天说“钟”,黑夜说“更”或“鼓”。又有“晨钟暮鼓”之说,古时城镇多设钟鼓楼,晨起(辰时,今之七点)撞钟报时,所以白天说“几点钟”;暮起(酉时,今之十九点)鼓报时,故夜晚又说是几鼓天。夜晚说时间又有用“更”的,这是由于巡夜人,边巡行边打击梆子,以点数报时。全夜分五个更,第三更是子时,所以又有“三更半夜”之说。 时以下的计量单位为“刻”,一个时辰分作八刻,每刻等于现时的十五分钟。旧小说有“午时三刻开斩”之说,意即,在午时三刻钟(差十五分钟到正午)时开刀问斩,此时阳气最盛,阴气即时消散,此罪大恶极之犯,应该“连鬼都不得做”,以示严惩。阴阳家说的阳气最盛,与现代天文学的说法不同,并非是正午最盛,而是在午时三刻。古代行斩刑是分时辰开斩的,亦即是斩刑有轻重。一般斩刑是正午
excel表格日期计算
竭诚为您提供优质文档/双击可除 excel表格日期计算 篇一:excel中几个时间计算公式 假设b2为生日 =datedif(b2,today(),"y") datediF函数,除excel2000中在帮助文档有描述外,其他版本的excel在帮助文档中都没有说明,并且在所有版本的函数向导中也都找不到此函数。但该函数在电子表格中确实存在,并且用来计算两个日期之间的天数、月数或年数很方便。微软称,提供此函数是为了与lotus1-2-3兼容。 该函数的用法为 “datediF(start_date,end_date,unit)”,其中start_date 为一个日期,它代表时间段内的第一个日期或起始日期。end_date为一个日期,它代表时间段内的最后一个日期或结束日期。unit为所需信息的返回类型。 “y”为时间段中的整年数,“m”为时间段中的整月数,“d”时间段中的天数。“md”为start_date与end_date日期中天数的差,可忽略日期中的月和年。“ym”为start_date 与end_date日期中月数的差,可忽略日期中的日和年。“yd”
为start_date与end_date日期中天数的差,可忽略日期中的年。比如,b2单元格中存放的是出生日期(输入年月日时,用斜线或短横线隔开),在c2单元格中输入 “=datedif(b2,today(),"y")”(c2单元格的格式为常规),按回车键后,c2单元格中的数值就是计算后的年龄。此函数在计算时,只有在两日期相差满12个月,才算为一年,假如生日是20xx年2月27日,今天是20xx年2月28日,用此函数计算的年龄则为0岁,这样算出的年龄其实是最公平的。 身份证号提取年龄 =datediF(--text((len(a1)=15)*19”即可获得当时的日期时间; 2、使用公式:用=now()而非=date(),=date()只有日期,然后进行菜单“工具->选项”,选择“重新计算”页,选中“人工重算”,勾不勾选“保存前自动重算”看自己的需要和想法了,如果勾选了,那日期时间那总是最后一次保存的日期时间,不勾选的话,如果你的表格中有公式记得准备存前按F9 篇二:excel中如何计算两个日期之间的月数 excel中如何计算两个日期之间的月数
时间计算公式
高中地理计算公式 一、时间的计算 1、求时区: 时区数=已知经度/15°(商四舍五入取整数,即为时区数) 2、求区时: 所求区时=已知区时±时区差(东加西减) 3、求地方时: 所求地方时=已知地方时±4分钟/度×经度差(东加西减) 二、太阳高度的计算 1、求正午太阳高度: H=90°-︱纬度差︱(纬度差指当地纬度与太阳直射纬度之间的差) 2、求子夜太阳高度: H=︱纬度和︱-90°(纬度和指当地纬度与太阳直射纬度之间的和) 3、求南北两楼的楼间距: L=h?cotH (h为楼高,H为该地一年中最小的正午太阳高度) 三、昼夜长短的计算 1、求昼长: (1)昼长=昼弧∕15° (2)昼长=日落时间-日出时间 (3)昼长=24-夜长 (4)昼长=(12-日出地方时)×2 (5)昼长=(日落地方时-12) 2、求夜长 (1)夜长=夜弧∕15° (2)夜长=24-昼长 (3)夜长=(24-日落地方时)×2 (4)北半球某纬度的夜长=南半球同纬度的昼长 四、日出、日落时刻的计算 1、求日出时刻: (1)日出时刻=当地纬线与晨线交点的时刻(2)日出时刻=12-昼长∕2 2、求日落时刻: (1)日落时刻=当地纬线与昏线交点的时刻(2)日落时刻=12+昼长∕2 五、球面距离的计算 (1)赤道和经线上的距离111Km×度数 (2)纬线上的距离=111Km×度数?COSθ(θ为当地的纬度) (3)对趾点的计算:经度互补,一东一西;纬度相等,一南一北。 六、比例尺的计算 (1)比例尺=图上距离∕实地距离 (2)缩放图幅面积=原图幅面积×比例尺缩放的平方 七、相对高度的计算 (1)陡崖相对高度:(n-1)d≤H<(n+1)d (n为等高线条数,d为等高距) (2)H=T∕6°×1000米 (H为两地相对高度,T为两地温差)
X射线光电子能谱的原理及应用(XPS)
X射线光电子能谱的原理及应用(XPS) (一)X光电子能谱分析的基本原理 X光电子能谱分析的基本原理:一定能量的X光照射到样品表面,和待测物质发生作用,可以使待测物质原子中的电子脱离原子成为自由电子。该过程可用下式表示: hn=Ek+Eb+Er 其中: hn:X光子的能量;Ek:光电子的能量;Eb:电子的结合能;Er:原子的反冲能量。其中Er很小,可以忽略。 对于固体样品,计算结合能的参考点不是选真空中的静止电子,而是选用费米能级,由内层电子跃迁到费米能级消耗的能量为结合能Eb,由费米能级进入真空成为自由电子所需的能量为功函数Φ,剩余的能量成为自由电子的动能Ek, 式(103)又可表示为:hn=Ek+Eb+Φ (10.4)Eb= hn- Ek-Φ (10.5) 仪器材料的功函数Φ是一个定值,约为4eV,入射X光子能量已知,这样,如果测出电子的动能Ek,便可得到固体样品电子的结合能。各种原子,分子的轨道电子结合能是一定的。因此,通过对样品产生的光子能量的测定,就可以了解样品中元素的组成。元素所处的化学环境不同,其结合能会有微小的差别,这种由化学环境不同引起的结合能的微小差别叫化学位移,由化学位移的大小可以确定元素所处的状态。例如某元素失去电子成为离子后,其结合能会增加,如果得到电子成为负离子,则结合能会降低。因此,利用化学位移值可以分析元素的化合价和存在形式。 (二)电子能谱法的特点 ( 1 )可以分析除H 和He 以外的所有元素;可以直接测定来自样品单个能级光电发射电子的能量分布,且直接得到电子能级结构的信息。 ( 2 )从能量范围看,如果把红外光谱提供的信息称之为“分子指纹”,那么电子能谱提供的信息可称作“原子指纹”。它提供有关化学键方面的信息,即直接测量价层电子及内层电子轨道能级。而相邻元素的同种能级的谱线相隔较远,相互干扰少,元素定性的标识性强。 ( 3 )是一种无损分析。 ( 4 )是一种高灵敏超微量表面分析技术。分析所需试样约10 -8 g 即可,绝对灵敏度高达10 -18 g ,样品分析深度约2nm 。 (三) X 射线光电子能谱法的应用 ( 1 )元素定性分析 各种元素都有它的特征的电子结合能,因此在能谱图中就出现特征谱线,可以根据这些谱线在能谱图中的位置来鉴定周期表中除H 和He 以外的所有元素。通过对样品进行全扫描,在一次测定中就可以检出全部或大部分元素。 ( 2 )元素定量分折 X 射线光电子能谱定量分析的依据是光电子谱线的强度(光电子蜂的面积)反映了原于的含量或相对浓度。在实际分析中,采用与标准样品相比较的方法来对元素进行定量分析,其分析精度达1 %~ 2 %。 ( 3 )固体表面分析 固体表面是指最外层的1 ~10 个原子层,其厚度大概是(0.1~1) n nm 。人们早已认识到在固体表面存在有一个与团体内部的组成和性质不同的相。表面研究包括分析表面的元素组成和化学组成,原子价态,表面能态分布。测定表面原子的电子云分布和能级结构等。X 射线 光电子能谱是最常用的工具。在表面吸附、催化、金属的氧化和腐蚀、半导体、电极钝化、薄膜材料等方面都有应用。 ( 4 )化合物结构签定 X 射线光电子能谱法对于内壳层电子结合能化学位移的精确测量,能提供化学键和电荷
日期计算器
程序设计与算法课程设计
课程设计评语 对课程设计的评语: 平时成绩:课程设计成绩: 总成绩:评阅人签名: 注:1、无评阅人签名成绩无效; 2、必须用钢笔或圆珠笔批阅,用铅笔阅卷无效; 3、如有平时成绩,必须在上面评分表中标出,并计算入总成绩。
目录 课程设计评语 (2) 目录 (3) 1.课程论文题目.............................................................................................. 错误!未定义书签。2.程序设计思路.. (4) 3.功能模块图 (4) 4.数据结构设计 (5) 5.算法设计 (5) 6.程序代码 (6) 7.程序运行结果 (7) 8.编程中遇到的困难及解决方法.................................................................. 错误!未定义书签。9.总结及建议.................................................................................................. 错误!未定义书签。10.致谢. (8)
1.课程设计题目:日期计算器 【要求】 功能:计算输入日期是当年中的第几天 系统要求实现以下功能: 1. 由用户分别输入:年、月、日 2. 计算该日期是当年中的第几天 3. 输出计算出的天数 分步实施: 1、首先设计Dater对象构造器 2、判断此年是否为闰年。 3、计算从此年年初到此日的一共多少天 4、输入输出处理。 【提示】 需求分析:使用Dater对象构造器,用1判断为闰年,,0判断为不是闰年,使用累加的方法计算年初到此日共有多少天,进行输入输出处理. (1)主函数设计 主函数提供输入,处理,输出部分的函数调用。 (2)功能模块设计 模块:由用户自己录入年,月,日,、。计算该日期为一年的中德第几天。输出计算出的天数,返回主菜单。 3. 功能模块图 (1)输入模块 由用户分别输入:年、月、日
X射线光电子能谱分析分析
一、X射线光电子能谱的测量原理 X射线光电子能谱(X-ray photoelectron Spectroscopy,简称XPS)也就是化学分析用电子能谱(Electron Spectroscopy for Chemical Analysis,简称ESCA),它是目前最广泛应用的表面分析方法之一,主要用于成分和化学态的分析。 用单色的X射线照射样品,具有一定能量的入射光子同样品原子相互作用,光致电离产生了光电子,这些光电子从产生之处输运到表面,然后克服逸出功而发射,这就是X射线光电子发射的三步过程。用能量分析器分析光电子的动能,得到的就是x射线光电子能谱。 根据测得的光电子动能可以确定表面存在什么元素以及该元素原子所处的化学状态,这就是x射线光电子谱的定性分析。根据具有某种能量的光电子数量,便可知道某种元素在表面的含量,这就是x射线光电子谱的定量分析。为什么得到的是表面信息呢?这是因为:光电子发射过程的后两步,与俄歇电子从产生处输运到表面然后克服逸出功而发射出去的过程是完全一样的,只有深度极浅范围内产生的光电子,才能够能量无损地输运到表面,用来进行分析的光电子能量范围与俄歇电子能量范围大致相同。所以和俄歇谱一样,从X射线光电子谱得到的也是表面的信息,信息深度与俄歇谱相同。 如果用离子束溅射剥蚀表面,用X射线光电子谱进行分析,两者交替进行,还可得到元素及其化学状态的深度分布,这就是深度剖面分析。 X射线电子能谱仪、俄歇谱仪和二次离子谱仪是三种最重要的表面成分分析仪器。X射线光电子能谱仪的最大特色是可以获得丰富的化学信息,三者相比,它对样品的损伤是最轻微的,定量也是最好的。它的缺点是由于X射线不易聚焦,因而照射面积大,不适于微区分析。不过近年来这方面已取得一定进展,分析者已可用约100 μm直径的小面积进行分析。最近英国VG公司制成可成像的X射线光电子谱仪,称为“ESCASCOPE”,除了可以得到ES-CA谱外,还可得到ESCA像,其空间分辨率可达到10μm,被认为是表面分析技术的一项重要突破。X射线光电子能谱仪的检测极限与俄歇谱仪相近,这一性能不如二次离子谱仪。 X射线光电子能谱的测量原理很简单,它是建立在Einstein光电发射定律基础之上的,对孤立原子,光电子动能E k为: E K =hv-E b. (12. 18) 这里hv是入射光子的能量,E b是电子的结合能。hv是已知的,E K可以用能量分析器测出,于是E b就知道了。同一种元素的原子,不同能级上的电子E b不同,所以在相同的hv 下,同一元素会有不同能量的光电子,在能谱图上,就表现为不止一个谱峰。其中最强而又最易识别的,就是主峰,一般用主峰来进行分析。不同元素的主峰,E b和E K不同,所以用能量分析器分析光电子动能,便能进行表面成分分析。 对于从固体样品发射的光电子,如果光电子出自内层,不涉及价带,由于逸出表面要克服逸出功φ,所以光电子动能为: E K=hv-Eb-φs (12. 19)
最新小学二年级钟表题时间计算题(经过时间计算)
精品好文档,推荐学习交流
二年级认识钟表练习----填经过时间
(
)
(
)
二年
班 姓名:
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
(
)
综合病例(过敏性休克)----场景一
仅供学习与交流,如有侵权请联系网站删除 谢谢1
(
)
(
)
精品好文档,推荐学习交流
题干内容
模拟病人设置
护理能力(考点)
(主动提供给选手)
(选手有询问或有操作责提 (本栏题干给评委)
供,反之则不提供)
1 床,李明,男,76 岁,体 1.病人:诉 5 分钟前做了青霉 1.评估:快速评估病人具体不
重 60KG,有中风病史 3 年,3 素皮试后,现全身皮肤瘙痒, 适,查看皮试的位置
天前因喂食发生呛咳后出现 头晕、胸闷、气促、呼吸困难 2.判断:口述病人可能出现过
发热、咳嗽、咳痰而入院, 伴濒死感。半卧位
敏性休克,需要立即抢救。
入院第二天,病人突然诉说 2.特殊用物准备:备皮试抢救 3.处理:
全身皮肤瘙痒,头晕、胸闷、 盒在床边
①呼救医生(特别考点)医生
气促、呼吸困难伴濒死感
3.家属:护士,我家人怎么 回复正在抢救另一个病人,尽
考核:病人可能发现什么情 了?为什么会这样?
快到达,考察选手抢救状态
况?你该如何处理
4.医生:呼救医生时,医生回 下,医生未到时的临床思维,
备注:开始 7 分钟,出现第 复正在抢救另一病人会尽快 继续操作得分,等待医生后操
二场景
过来
作扣分。
5.工作人员:第 7 分钟给选手 ②选手与家属有效沟通,人文
第二场景
关怀
③摆放平卧体位
④医生未到之前抽吸肾上腺
素 1 支 1mg(特别考点)指
出准备的剂量有错
⑤吸氧(中流量吸氧)
⑥心电监护(特别考点)因右
侧肢体瘫痪,原则上不用右侧
肢体绑袖带
场景二 题干内容 (主动提供给选手)
医生到达,口头医嘱肾上腺 素 1mg 皮下注射 考核要求:请执行医嘱并给 予相应的处理 备注:开始 9 分钟,出现第 三场景
模拟病人设置
护理能力(考点)
(选手有询问或有操作责提 (本栏题干给评委)
供,反之则不提供)
1.工作人员:选手上心电监护 4.向医生汇报病人情况
后提供:
5.肾上腺素 1mg 皮下注射
心电监护显示:HR130 次/分, R:28 次/分 BP:75/55mmHg, sop2:90% 2.工作人员:第 9 分钟给选手 第三场景
仅供学习与交流,如有侵权请联系网站删除 谢谢2
相关文档
- 时间计算表格
- 定时器定时时间计算
- 日期计算器
- 小学二年级钟表题时间计算题(经过时间计算)
- 充电电池时间计算表
- 小学三年级数学《简单的时间计算》教案范文三篇
- 蒸发时间推算表
- 一个表格中含有日期和时间,如何利用excel公式提炼出日期
- 最新小学二年级钟表题时间计算题(经过时间计算)
- 考勤表、日历、时间全自动计算生成
- Takt Time Calculator节拍时间计算器
- 机加时间计算表
- 阿米巴经营单位时间核算表
- 单位时间核算表
- 时间价值计算表
- 2018年考勤表(带公式自动计算-日期-星期自动)
- 单位时间核算表 (1)
- 阿米巴经营单位时间核算表
- 钟表题时间计算题(经过时间计算)
- 凝结时间计算表
