Carboncoated nano Si dispersed oxides graphite composites as anode material for lithiumion batteries

Carbon-coated nano-Si dispersed oxides/graphite composites
as anode material for lithium ion batteries
Heon-Young Lee,Sung-Man Lee
*
Department of Advanced Materials Science and Engineering,Kangwon National University,Chuncheon,Kangwon-Do 200-701,Republic of Korea
Received 13February 2004;received in revised form 9March 2004;accepted 9March 2004
Published online:9April 2004
Abstract
A carbon-coated nano-Si dispersed oxides/graphite composite has been prepared and investigated as a potential anode material for lithium ion batteries.The nano-Si dispersed oxides were synthesized by mechanochemical reduction of SiO by Al,and carbon has been coated onto the ball milled mixture of nano-Si dispersed oxide/graphite by utilizing pyrolysis of coal–tar pitch at 900°C in an argon ?ow.The carbon-coated composites show excellent cycling performance with a reasonable value of the ?rst irreversible capacity.The superior electrochemical characteristics are attributed to the uniform distribution of nano-sized Si phase,the bu?ering action of graphite and an enhanced connection for electronic and ionic conduction by carbon-coating.ó2004Elsevier B.V.All rights reserved.
Keywords:Lithium ion battery;Mechanochemical reduction;Anode materials;Carbon-coating
1.Introduction
Recently,there has been considerable interest in ?nding new electrode materials for a new generation of lithium ion batteries,which might have high energy density compared with the existing system.Silicon is attractive as an alternative anode material due to its very large lithium insertion capacity.However,the large volume change during charge and discharge reactions results in poor cyclability.Many researchers,therefore,have focused on improving the cycle performance of Si-based systems [1–8].It has been reported that carbon-coated silicon,prepared by coating carbon onto the surface of silicon particles using thermal vapor deposi-tion method,shows reversible capacity as high as 1000mA h/g and improved cycle stability under controlled lithium insertion conditions [4,5].Our previous study showed that the cyclability of the Si alloys was signi?-cantly improved by mechanical mixing with graphite [9].
Based on these studies,it seems to be concluded that the composite structure including active Si phase may be optimized when nano-sized Si elements are dispersed uniformly in a ductile inactive matrix and mixed with graphite powders,followed by carbon-coating.Here,the carbon-coating can be simply done by pyrolysing the carbon precursors to be carbonized through a liquid phase with nano-Si dispersed oxide/graphite mixture [10].Therefore,preparation of nano-Si/inactive matrix composite particles is a key factor in developing the carbon-coated nano-Si composite material.It is believed that reduction of metal oxides by mechanical alloying method is an ideal method to homogeneously disperse Si ?ne particles in situ into oxide matrix [11,12].During ball milling the mixture of SiO (or SiO 2)and Al pow-ders,the mechanically driven reduction reaction of SiO (or SiO 2)+Al !Si +Al x O y can be induced,which leads to a nano-Si/oxide composite particles.This method was applied to obtain nano-Si dispersed oxide particles.In the present study,we have developed a carbon-coated nano-Si dispersed oxide/graphite composite ma-terial and investigated its electrochemical performance as anode material for lithium ion batteries.
*
Corresponding author.Tel.:+82-33-250-6266;fax:+82-33-242-6256.
E-mail address:smlee@kangwon.ac.kr (S.-M.Lee).1388-2481/$-see front matter ó2004Elsevier B.V.All rights reserved.
doi:10.1016/j.elecom.2004.03.005
Electrochemistry Communications 6(2004)
465–469
https://www.sodocs.net/doc/9e320013.html,/locate/elecom
2.Experimental
The nano-Si dispersed oxide particles were prepared by mechanical ball milling the mixture of SiO(or SiO2), Al and Li2O2powders using a SPEX-8000high-energy mechanical https://www.sodocs.net/doc/9e320013.html,mercial elemental powders of SiO (Aldrich–325mesh),Al(99%)and Li2O2(99%)were used as starting materials.The starting materials were weighted(SiO:Al:Li2O2?1:1:0.2in molar ratio),mixed and loaded into a stainless-steel vial containing stainless-steel balls.All the process were conducted inside the glove box?lled with argon.
Carbon-coated Si dispersed oxide/graphite composite material was prepared as follows.The Si dispersed oxide particles obtained by mechanical reduction process were mixed(1:1by weight)with graphite and then the mix-ture was milled with coal–tar pitch followed by heating at900°C for1h under argon atmosphere.The carbon-coated product was ground using variable speed rotor-mill(Fritch,pulverisette14)to control the size and shape of the composite material.The sample was char-acterized by X-ray di?raction(XRD)with Cu-K a radi-ation to identify the phases formed.Morphology of the sample was observed by a scanning electron microscope (SEM).
Electrodes were prepared by pasting a slurry con-taining85wt%active materials,5wt%carbon black and 10wt%polyvinylidene?uoride(PVDF)as a binder, dissolved in N-methyl-2-pyrrolidone(NMP)onto a copper mesh.The electrodes were then dried overnight
at120°C under vacuum and then pressed.The elec-trolyte was1M LiPF6in a mixture of ethylene car-bonate(EC)and diethyl carbonate(DEC)(1:1by volume,provided by Cheil Industries Inc.,South Ko-rea).Half-cells were assembled in an argon-?lled glove-box and galvanostatically charged and discharged in the voltage range0.0–1.2V vs.Li/Litusing a current den-sity of0.2mA/cm2.
3.Results and discussion
Preliminary experiments showed that the addition of Li2O2improved the electrochemical properties such as the cycle life of the resulting electrode.Hence,we in-cluded Li2O2in starting mixture for the mechano-chemical reduction reaction,although the exact function of Li2O2is not clear at present.
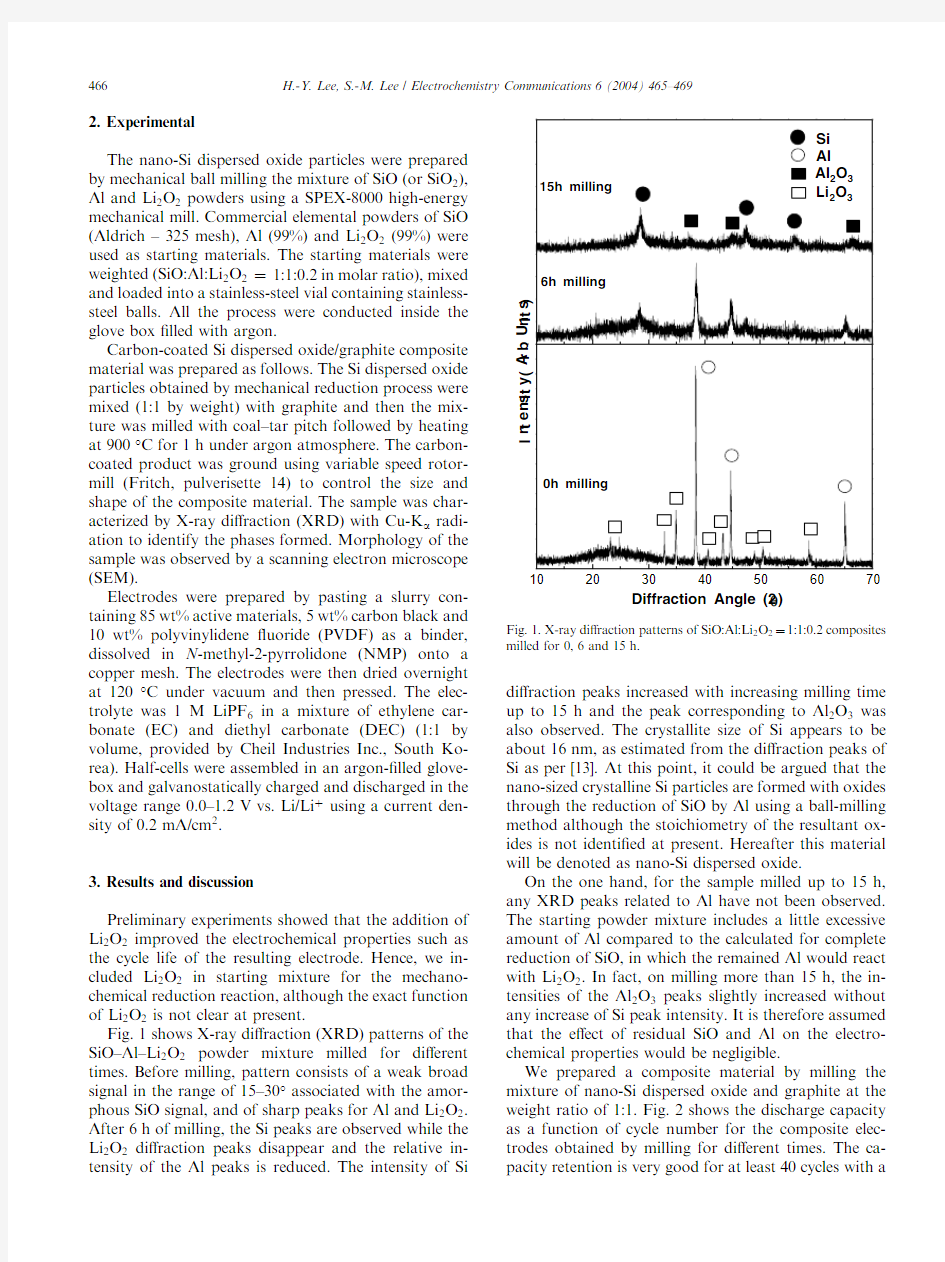
Fig.1shows X-ray di?raction(XRD)patterns of the SiO–Al–Li2O2powder mixture milled for di?erent times.Before milling,pattern consists of a weak broad signal in the range of15–30°associated with the amor-phous SiO signal,and of sharp peaks for Al and Li2O2. After6h of milling,the Si peaks are observed while the Li2O2di?raction peaks disappear and the relative in-tensity of the Al peaks is reduced.The intensity of Si di?raction peaks increased with increasing milling time up to15h and the peak corresponding to Al2O3was also observed.The crystallite size of Si appears to be about16nm,as estimated from the di?raction peaks of Si as per[13].At this point,it could be argued that the nano-sized crystalline Si particles are formed with oxides through the reduction of SiO by Al using a ball-milling method although the stoichiometry of the resultant ox-ides is not identi?ed at present.Hereafter this material will be denoted as nano-Si dispersed oxide.
On the one hand,for the sample milled up to15h, any XRD peaks related to Al have not been observed. The starting powder mixture includes a little excessive amount of Al compared to the calculated for complete reduction of SiO,in which the remained Al would react with Li2O2.In fact,on milling more than15h,the in-tensities of the Al2O3peaks slightly increased without any increase of Si peak intensity.It is therefore assumed that the e?ect of residual SiO and Al on the electro-chemical properties would be negligible.
We prepared a composite material by milling the mixture of nano-Si dispersed oxide and graphite at the weight ratio of1:1.Fig.2shows the discharge capacity as a function of cycle number for the composite elec-trodes obtained by milling for di?erent times.The ca-pacity retention is very good for at least40cycles with a
466H.-Y.Lee,S.-M.Lee/Electrochemistry Communications6(2004)465–469
reversible capacity over600mA h/g.However,the initial capacity loss is considerable,which increases with mill-ing time for the preparation of the composites.This ir-reversible capacity occurs mainly near0.8V as shown in Fig.3,which is caused by side reactions with electrolyte at the surfaces of the graphite[14,15].It has been found that carbon-coating onto graphite particles is very ef-fective in reducing the?rst irreversible capacity associ-ated with the surfaces of graphite particles[10,16–18].
Fig.4shows the XRD pattern of carbon-coated composite powder which was prepared by heating the mixture of composite material and coal–tar pitch(70 wt%)at900°C in an argon?ow.For comparison,the XRD pattern of the composite powders before carbon-coating is also given.It is worthwhile to note that the broad signal of Si peak remained even after heat treat-ment for carbon-coating,while di?raction peaks for Al2O3became visible.This indicates that the carbon-coated composite material includes nano-sized Si.Here the Si phase is truly nano-crystalline( 20nm)as de-termined from peak broadening analysis[13].
Fig.5shows SEM micrographs of the nano-Si dis-persed oxide,the nano-Si dispersed oxide/graphite composite and the carbon-coated composite.In the case of nano-Si dispersed oxide/graphite composite,oxide particles were uniformly distributed in the graphite. Notice that the carbon-coated composite was obtained by manual grinding followed by milling using a speed rotor-mill at12000rpm,which leads to the formation of spherical,agglomerated particles.The?rst cycle dis-charge–charge curves of the carbon-coated composite electrode is given in Fig.6.As expected,the irreversible capacity is signi?cantly reduced by carbon-coating treatment.
It can be also seen that the plateau at around0.45V in the charge curve is more visible after the carbon-coating treatment.This plateau is due to the Li extrac-tion from the Li x Si alloy[5].It can be considered that the enhanced plateau is attributed to the increase of Si crystal size after carbon-coating treatment,as measured from XRD data.
The cycling performance is also further improved compared with that for bare composite of Fig.2,as shown in Fig.7.These results demonstrate an evident e?ect of carbon-coating treatment on the improvement of the electrochemical performance of nano-Si dispersed oxide/graphite composite.The coal–tar pitch carbon-coating e?ectively depresses the irreversible side reactions by covering the active sites for electrolyte de-composition.Moreover,the resultant powder shows a spherically aggregated shape with reduced speci?c sur-face area.As a result of carbon-coating treatment,it is also supposed that the core active material of nano-Si
H.-Y.Lee,S.-M.Lee/Electrochemistry Communications6(2004)465–469467
based oxide is more closely connected with graphite particles,and the electrical conductivity of the com-posite material is enhanced.In situ formed inactive ox-ides and the graphite act as a bu?ering matrix for Li–Si alloying of nano-Si active material during cycling.Therefore,the excellent electrochemical performance is attributed to the combined e?ects of the uniform dis-tribution of nano-Si phase in inactive oxides,the graphite mixing and the carbon coating.
4.Conclusions
A nano-Si dispersed oxide is synthesized by mecha-nochemical reduction induced by ball milling of the powder mixture of SiO and Al.The composites,ob-tained by ball milling the nano-Si dispersed oxide/graphite mixture,show a stable capacity retention dur-ing cycling,but ball milling of graphite leads to initial irreversible capacity loss.It appeared that carbon-coat-
Fig.5.Scanning electron micrographs of (a)nano-Si dispersed oxide,(b)nano-Si dispersed oxide/graphite composite,(c)carbon-coated nano-Si dispersed oxide/graphite composite obtained by manual grinding after carbon-coating,(d)carbon-coated nano-Si dispersed oxide/graphite com-posite,manually ground and then milled by the speed rotor-mill.
468H.-Y.Lee,S.-M.Lee /Electrochemistry Communications 6(2004)465–469
ing treatment on the composites provides improved cy-cling ability of the composites with reduced irreversible capacity.The superior properties are attributed to the combined e?ects of the uniform distribution of nano-Si phase in inactive oxides,the graphite mixing and the carbon-coating.
Acknowledgements
This research was supported by University IT Re-search Center Project.
References
[1]H.Li,X.Huang,Li.Chen,Z.Wu,Y.Liang,Electrochem.Solid
State Lett.2(1999)547.
[2]I.S.Kim,P.N.Kumta,G.E.Blomgren,Electrochem.Solid State
Lett.3(2000)493.
[3]S.M.Hwang,H.Y.Lee,S.W.Jang,S.M.Lee,S.J.Lee,H.K.Baik,
J.Y.Lee,Electrochem.Solid State Lett.4(2001)97.
[4]M.Yoshio,H.Wang,K.Fukuda,T.Umeno,N.Dimov,Z.
Ogumi,J.Electrochem.Soc.149(2002)1598.
[5]N.Dimov,S.Kugino,M.Yoshio,Electrochim.Acta48(2003)
1579.
[6]J.Yang, B.F.Wang,K.Wang,Y.Liu,J.Y.Xie,Z.S.Wen,
Electrochem.Solid State Lett.6(2003)A154.
[7]H.Dong,X.P.Ai,H.X.Yang,https://www.sodocs.net/doc/9e320013.html,mun.5(2003)
952.
[8]X.W.Zhang,P.K.Patil,C.Wang,A.J.Appleby,F.E.Little,D.L.
Cocke,J.Power Sources125(2004)206.
[9]H.Y.Lee,S.M.Lee,J.Power Sources112(2002)649.
[10]H.Y.Lee,J.K.Baek,S.W.Jang,S.M.Lee,S.T.Hong,K.Y.Lee,
M.H.Kim,J.Power Sources101(2001)206.
[11]J.M.Wu,Z.Z.Li,J.Alloys Compd.299(2000)9.
[12]K.Tokumitsu,Solid State Ionics101–103(1997)25.
[13]C.Suryanarayana,M.Grant Norton,X-ray Di?raction:A
Practical Approach,Plenum Press,New York,1998,p.207. [14]R.Fong,U.Von Sacken,J.R.Kahn,J.Electrochem.Soc.137
(1990)2009.
[15]M.Winter,P.Novak, A.Monnier,J.Electrochem.Soc.145
(1998)428.
[16]I.Kuribayashi,M.Yokoyama,M.Yamashita,J.Power Sources
54(1995)1.
[17]W.Qiu,G.Zhang,S.Lu,Q.Liu,Solid State Ionics121(1999)73.
[18]M.Yoshio,H.Wang,K.Fukuda,Y.Hara,Y.Adachi,J.
Electrochem.Soc.147(2000)1245.
H.-Y.Lee,S.-M.Lee/Electrochemistry Communications6(2004)465–469469
