3.0-Anti-inflammatory effects of fangchinoline and tetrandrine

Journal of Ethnopharmacology69(2000)173–179
Anti-in?ammatory effects of fangchinoline and tetrandrine Hong-Serck Choi a,Hack-Seang Kim a,*,Kyung Rak Min a,Youngsoo Kim a, Hwa Kyung Lim a,Young Kyung Chang b,Myeon Woo Chung c
a College of Pharmacy,Chungbuk National Uni6ersity,Cheongju,Chungbuk361-763,South Korea
b Di6ision of Cosmetics and Hygieni
c Products,Department of Drug E6aluation,Korea Foo
d and Drug Administration,
Seoul,South Korea
c National Institute of Toxicological Research,Korea Foo
d and Drug Administration,Seoul,South Korea
Received6June1999;received in revised form14July1999;accepted28July1999
Abstract
Fangchinoline and tetrandrine are the major alkaloids from Stephania tetrandrae S.Moore which has been used traditionally for the treatment of in?ammatory diseases in oriental countries including Korea.Both fangchinoline and tetrandrine showed anti-in?ammatory effects on mouse ear edema induced by croton oil.In addition,the effects of fangchinoline and tetrandrine on cyclooxygenase,murine interleukin-5(mIL-5)and human interleukin-6(hIL-6)were examined in vitro to investigate the anti-in?ammatory action mechanisms.One hundred micromolar of fangchinoline showed35%of inhibition on cyclooxygenase,but the same concentration of tetrandrine did not show any inhibition. On the other hand,12.5m M of tetrandrine exhibited95%of inhibition on mIL-5activity,while fangchinoline did not show any effects.However,4m M of fangchinoline and6m M of tetrandrine showed63and86%of inhibitions on hIL-6activity,respectively.These results suggest that biochemical mechanisms of fangchinoline and tetrandrine on anti-in?ammation are signi?cantly different even though they are similar in chemical structure.?2000Elsevier Science Ireland Ltd.All rights reserved.
Keywords:Fangchinoline;Tetrandrine;Anti-in?ammation;Mouse ear edema;Cyclooxygenase;mIL-5;hIL-6
https://www.sodocs.net/doc/8d17259844.html,/locate/jethpharm
1.Introduction
Fangchinoline and tetrandrine(Fig.1)are the major alkaloids of the tuberous root of the creeper,Stephania tetrandrae S.Moore of the Menispermaceae family(Huang,1993).They have been used traditionally for the treatment of in?-ammatory diseases in China.Tetrandrine has been known to have inhibitory effects on silicosis and asthma(Li et al.,1981;Liu et al.,1983;Tsai et al.,1995),but its biochemical mechanism has not yet been known clearly.Fangchinoline is known to have inhibitory effects on histamine release(Nakamura et al.,1992)and on the pro-duction of IL-1and tumor necrosis factor-a (TNF-a)(Onai et al.,1995).It is reported that fangchinoline had less effect on in?ammatory in-hibition than tetrandrine(Lu et al.,1957).In
*Corresponding author.Tel.:+82-431-261-2813;fax:+
82-431-268-2732.
E-mail address:hskim@trut.chungbuk.ac.kr(H.-S.Kim)
0378-8741/00/$-see front matter?2000Elsevier Science Ireland Ltd.All rights reserved. PII:S0378-8741(99)00141-5
H.-S.Choi et al./Journal of Ethnopharmacology69(2000)173–179 174
addition,fangchinoline(Kim et al.,1997)and tetrandrine(Kim et al.,1997;King et al.,1988) have been demonstrated as nonspeci?c calcium channel antagonists.However,the action mecha-nisms of anti-in?ammation of fangchinoline and tetrandrine have not also been fully understood. Therefore,the present study was undertaken to investigate the anti-in?ammatory effects of fangchinoline and tetrandrine administered orally and applied topically on croton oil induced mouse ear edema.In addition,the effects of fangchino-line and tetrandrine on cyclooxygenase,mIL-5 and hIL-6activity in vitro were also examined to investigate the anti-in?ammatory action mechanisms.
2.Materials and methods
2.1.Materials
Fangchinoline and tetrandrine were isolated from the creeper Stephania tetrandrae S.Moore (or fenfangji)and con?rmed by comparing the physical and chemical properties such as mp and TLC with standard compounds and1H-NMR spectra with the reports(Yamaguchi,1970;Lin et al.,1993).The roots of Stephania tetrandrae S. Moore were collected in Hebei province(China) and identi?ed by Professor Yong-Zhen Liu,De-partment of Pharmacology,College of Pharmacy, Yanbian University(Yanji,China).A voucher specimen was deposited in the same University. The following chemicals were used:dexam-ethasone(Youngin Pharmaceuticals),Tween80, croton oil,bovine hemoglobin,arachidonic acid, sodium sul?te,dimethylsulfoxide,RPMI-1640, Hanks’balanced salts,2-mercaptoethanol, methotrexate,3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide(MTT)(Sigma,St Louis,MO),acetone(Jin Chemical and Pharma-ceutical),sheep seminal vesicles(Pel-Freeze Bio-logicals),Tris,EDTA(Kodak International Biotechnologies),phenol(Boehringer Mannheim), indomethacin(Hyundai Pharmaceuticals),O2 probe solution(YSI),ethanol(Hayman),fetal bovine serum(Gilbco BRL Life Technologies), benzylpenicillin potassium,streptomycin sulfate,trypan blue(Wako Chemical),2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo-phenyl)-2H-tetra-zolium(WST-1),1-methoxy-5-methylphenaznium methylsulfate(1-methoxy PMS)(Dojindo Labora-tories)and trypsin(Difco Laboratories).
2.2.Animals
ICR male mice weighing20–25g were pur-chased from Sam Yuk Laboratory Animal(Osan, Korea).They were housed in an acryl?ber cage in a controlled room(temperature2293°C) and were maintained on a12h light/dark cycle. They were given a solid diet and tap water ad libitum.
2.3.Ear edema test
In vivo anti-in?ammatory effects of fangchino-line and tetrandrine were evaluated using mouse ear edema test as an acute in?ammation model according to the slightly modi?ed method of Ton-neli et al.(1965).In order to measure the systemic anti-in?ammatory effects of fangchinoline and te-trandrine,they were?nely suspended in10% Tween80(4ml/kg)and administered orally.Five percent croton-oil was then dissolved in acetone (25m l/ear)and applied topically1h later(Table 1).
Ear thickness was measured with a dial thick-ness gauge(Ozaki MFG,Japan)5h after the croton-oil treatment.
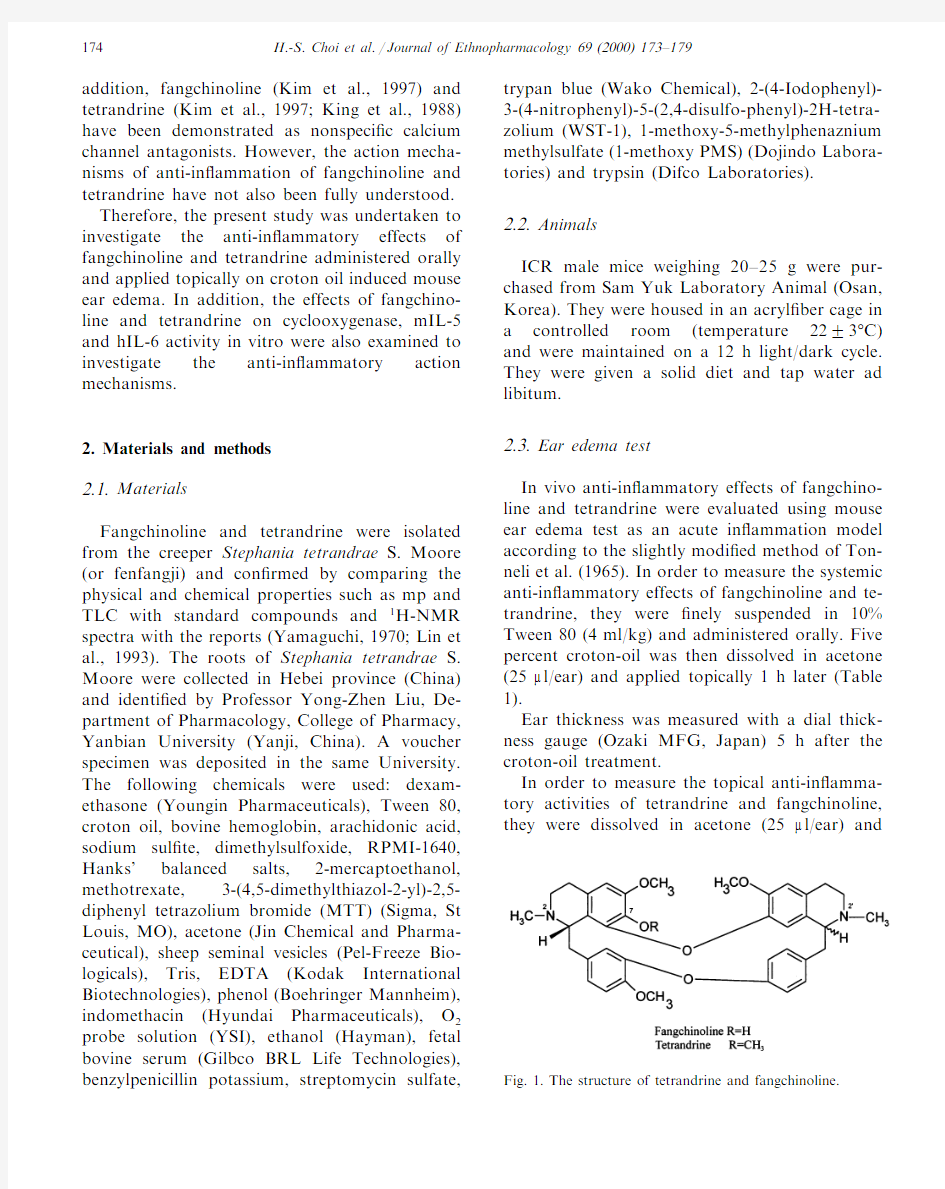
In order to measure the topical anti-in?amma-tory activities of tetrandrine and fangchinoline, they were dissolved in acetone(25m l/ear)and Fig.1.The structure of tetrandrine and fangchinoline.
H.-S.Choi et al./Journal of Ethnopharmacology69(2000)173–179175
Table1
Inhibitory effects of tetrandrine and fangchinoline adminis-tered orally on croton-oil induced ear edema
Dose(mg/kg)Thisckness increased a Drugs
(Mean9S.D.) Fangchinoline0.2690.02(0)b
Control
0.1890.01(27)**
80
0.1990.01(26)**
40
200.2090.01(24)* Tetrandrine0.2790.00(0)b
Control
0.1890.01(33)**
80
0.1790.01(38)**
40
200.2190.01(23)** Indomethacin0.2890.02(0)b
Control
800.1890.02(37)**
a Scale,mm.All compounds were orally administered(n=
7).
b Inhibitory effects are represented as%of inhibition and signi?cances of them are*P B0.01and**P B0.001.creased-sample thickness increased)/(control thickness increased)]×100.
2.4.Cyclooxygenase assay
Fangchinoline and tetrandrine were dissolved into100%DMSO to make stock solutions and subsequent dilutions were also made in100% DMSO.
Enzyme sources used were microsomal frac-tions prepared from sheep seminal vesicles.All of the following steps were executed at4°C.Sheep seminal vesicles were homogenized with a poly-tron homogenizer in5–10volumes of0.1M Tris–HCl(pH8.0).The homogenate was cen-trifuged at10000×g for10min,and the result-ing supernatant was centrifuged again at 100000×g for1h.The precipitated microsome was homogenized with a glass homogenizer in0.1 M Tris–HCl(pH8.0)at1–10mg/ml and was used as the enzyme source of prostaglandin H2 synthase-1(PGHS-1).Cyclooxygenase activity of the enzyme was measured oxygen consumption using a oxygen monitor(YSI)as described previ-ously(Min et al.,1996).A typical assay mixture contained3ml of0.1M Tris–HCl(pH8.0),50m l of arachidonic acid(2ng/ml),25m l of hemoglobin (3.4mg/1ml of0.1M Tris–HCl(pH8.0)-1mM phenol)and60m l of sample.Bovine hemoglobin was used as the source of heme which is coenzyme of PGHS-1.Enzyme reactions were initiated by adding the50m l of source of enzyme.The in-hibitory effects of fangchinoline and tetrandrine on cyclooxygenase activity were expressed as per-cent of inhibition.
[(control O2?sample O2)/(control O2)]×100 where O2means the initial rate of oxygen consumption.
2.5.Cell culture
Cells were grown at37°C in a humidi?ed atmo-sphere(5%CO2/95%air)in RPMI medium (RPMI-164010.4g/l,24mM NaHCO3,100m g/ml benzylpenicillin potassium,100m g/ml strepto-mycin sulfate(pH7.1),50m M2-mercaptoethanol) containing8%inactivated FBS with mIL-5(Y16) or10%inactivated FBS with hIL-6(MH60).
applied to the mices’ears.After30min,the5% croton-oil was topically applied to the mices’ears and the thickness was measured5h later (Table2).The anti-in?ammatory effects of fangchinoline and tetrandrine were expressed as percent of inhibition.[(control thickness in-Table2
Inhibitory effects of tetrandrine and fangchinoline applied topically on croton-oil induced ear edema a
Drugs Thickness increased a
Dose(mg/ear)
(Mean9S.D.)
Fangchinoline Control0.2490.02(0)b
0.1790.02(29)***
0.1
0.050.1990.02(24)**
0.0250.2090.02(24)*
Tetrandrine Control0.2790.02(0)b
0.10.2090.01(24)**
0.050.2190.02(23)**
0.0250.2390.01(13)*
0.2790.01(0)b Dexamethasone Control
0.10.0590.01(83)**
0.010.0890.01(71)**
0.0010.1390.01(51)**
a Scale,mm.All compounds were topically applied(n=7).
b Inhibitory effects are represented as%of inhibition and signi?cances of them are*P B0.05,**P B0.005and***P B 0.001.
H .-S .Choi et al ./Journal of Ethnopharmacology 69(2000)173–179
1762.6.Preparation of the sample solution
After tetrandrine and fangchinoline were dis-solved in 10mM in 100%DMSO,they were diluted to 1mM in RPMI-8%FBS.Then they were ?ltrated with a nitrocellulose ?lter (pore size 0.2m m).When in use,they were diluted to proper concentration in RPMI-8%FBS (mIL-5bioassay)or RPMI-10%FBS (hIL-6bioassay).The ?nal concentration of DMSO was B 0.1%.
2.7.Inhibition on mIL -5bioassay
The Y16cell used in this study is the early B cell which proliferates in depending on mIL-5.The Y16cell was obtained from professor K.Takatsu (Tokyo University,Japan).Y16cells were washed and resuspended in RPMI-8%FBS at a concen-tration of 1×104cells /ml.After 5U /ml of mIL-5was added to the cells,Y16cells were cultured in a humidi?ed atmosphere containing 5%CO 2at 37°C for 2days.After 2days culture,Y16cells were washed with Hanks’solution,resuspended in RPMI-8%FBS and adjusted to a concentration of 1×105cells /ml.To each well of a 96-well mi-croplate was delivered 100m l of the cells,50m l of mIL-5(1U /ml as the ?nal concentration)and 50m l of samples.The microplate was incubated in a humidi?ed atmosphere containing 5%CO 2at 37°C for 2days.Y16cell proliferation was mea-sured by using a WST-1colorimetric assay (Kawase et al.,1995)with the microplate reader.After 20m l 1-methoxy PMS:WST-1mixture solu-tion was added per well in a 96-well microplate and kept in 5%CO 2at 37°C for 4h,Y16cell proliferation was measured with the microplate reader.The optical density was at 450nm in contrast with 690nm.Percent of inhibition was calculated using the following formula:Percent of inhibition =
!
1?(sample OD 450?blank OD 450)
(control OD 450?blank OD 450)
"
×100
2.8.Inhibition on hIL -6bioassay
The MH60cell used in this study is the murine B cell hybridoma cell which proliferates in de-
pending on hIL-6.MH60cells were obtained from professor T.Hirano (Osaka University,Japan).MH60cells were washed and resuspended in RPMI-10%FBS at a concentration of 1×104cells /ml.After 1U /ml of hIL-6was added to the cells,the cells were cultured in a humidi?ed atmo-sphere containing 5%CO 2at 37°C for 2days.After 2days culture,the cells were washed with Hanks’solution,resuspended in RPMI-10%FBS and then adjusted to a concentration of 1×105cells /ml.To each well of a 96-well microplate was delivered 100m l of the MH60cells,50m l of hIL-6(0.3U /ml as the ?nal concentration)and 50m l of samples.The microplate were incubated in a humidi?ed atmosphere containing 5%CO 2at 37°C for 2days.MH60cell proliferation was measured using the MTT test (Mossman,1983).After 20m l MTT solution was added per well in a 96-well microplate and kept in 5%CO 2at 37°C for 4h,the microplate was centrifuged at 2000rpm for 10min.Supernatant was removed and 150m l 0.01N HCl /isopropanol were added to a 96-well microplate.After the precipitate was dis-solved,MH60cell proliferation was measured with the microplate reader.The optical density was at 570nm,in contrast with 690nm.Percent of inhibition was calculated using the following formula:
Percent of inhibition =
!
1?(sample OD 450?blank OD 450)
(control OD 450?blank OD 450)
"
×100
2.9.Statistical analysis
Data were expressed as mean 9S.E.of the mean and the signi?cance was analyzed by Stu-dent’s t -test.
3.Results
3.1.Ear edema test
Fangchinoline at doses of 20,40and 80mg /kg administered orally showed 24,26and 27%of inhibitions of croton oil induced ear edema,re-spectively,compared with that of control group.
H.-S.Choi et al./Journal of Ethnopharmacology69(2000)173–179177
Table3
Inhibitory effects of fangchinoline and tetrandrine on cy-clooxygenase activity a
Drugs%of
Dose(m M)IC50(m M)
inhibition
150
Fangchinoline6090.8**129
3594.1*
100
2491.2*
50
B0
Tetrandrine\150
150
100B0
B0
50
a Inhibitory effects are represented as%of inhibition, mean9S.E.(n=3),and signi?cances of them are**P B0.001 and*P B0.01.Therefore a concentration of1U/ml of mIl-5was chosen in order to investigate the inhibitory effects of fangchinoline and tetrandrine on mIL-5activity. It was observed that tetrandrine suppressed mIL-5-dependent Y16proliferation(Table4).But, fangchinoline did not.12.5,25and50m M of tetrandrine showed more than95%of inhibitions, but6m M of tetrandrine showed43%of inhibition. The IC50of tetrandrine on mIL-5activity is9.9m M.
3.4.The inhibitory effects of tetrandrine and fangchinoline on hIL-6acti6ity
In a preliminary experiment,0.3U/ml of hIL-6 showed submaximal proliferation of MH60cells. Therefore a concentration of0.3U/ml of hIl-6was chosen in order to investigate the inhibitory effects of fangchinoline and tetrandrine on hIL-6activity. Both tetrandrine and fangchinoline signi?cantly inhibited hIL-6-dependent MH60proliferation (Table5). 2.5m M and4m M of fangchinoline showed17and63%of inhibitions respectively,and 5m M of fangchinoline showed more than95% inhibition.The IC50of fangchinoline on hIL-6 bioassay is 3.7m M,and 2.5,5and6m M of tetrandrine showed3,32and86%of inhibitions respectively,and7m M of tetrandrine showed more than95%of inhibition,and the IC50of tetrandrine is5.7m M.
In addition,20,40and80mg/kg of tetrandrine administered orally showed23,38and33%of inhibitions on croton oil induced ear edema, respectively,compared with that of control group. Especially,40mg/kg of tetrandrine showed inhibitory effects which is similar to that of80 mg/kg of indomethacin.
Less than0.05mg/ear of fangchinoline applied topically showed24%of inhibitions and0.1 mg/ear of fangchinoline29%of inhibition on croton oil induced ear edema,respectively, compared with that of control group.In addition, 0.025,0.05and0.1mg/ear of tetrandrine applied topically showed13,23and24%of inhibitions on
croton oil induced ear edema,respectively, compared with that of control group.
3.2.Inhibitory effects of tetrandrine and fangchinoline on cyclooxygenase acti6ity
A concentration of5,100and150m M fangchi-noline showed24,35and60%of inhibitions of cyclooxygenase activity,respectively.The50%of inhibition(IC50)of fangchinoline on cyclooxyge-nase activity is129m M(Table3).
Tetrandrine did not show any inhibitory effects from50to150m M concentrations at all.
3.3.Inhibitory effects of tetrandrine and fangchinoline on mIL-5acti6ity
In a preliminary experiment,1U/ml of mIL-5 showed submaximal proliferation of Y16cells.Table4
Inhibitory effects of fangchinoline and tetrandrine on mIL-5 activity a
IC50(m M) Drugs%of
Dose(m M)
inhibition
50\150 Fangchinoline B0
25B0
12.5B0
B0
6
3B0
50
Tetrandrine\9590.1*9.9
25\9590.1*
12.59591.2*
64393.3*
3B092.1**
a Inhibitory effects are represented as%of inhibition, mean9S.E.(n=3),and signi?cances of them are*P B0.001 and**P B0.5.
H.-S.Choi et al./Journal of Ethnopharmacology69(2000)173–179 178
Table5
Inhibitory effects of fangchinoline and tetrandrine on hIL-6 activity a
%of
Dose(m M)
Drugs IC50(m M)
inhibition
10
Fangchinoline\9590.4*** 3.7
\9590.2***
7.5
\9593.7***
5
6398.9c
4
2695.1c
3
1795.8*
2.5
\9590.9*** Tetrandrine 5.7
10
\9590.8***
7.5
\9590.9***
7
8693.0***
6
3394.5**
5
3.492.2*
2.5
a Inhibitory effects are represented as%of inhibition, mean9S.E.(n=3),and signi?cances of them are***P B 0.001,c P B0.005;**P B0.01and P B0.1*.et al.,1995).But the exact mechanisms of anti-?amation by fangchinoline and tetrandrine re-main unclear.Even though IL-5,IL-6and IL-1 differ in some properties,these cytokines share various common biological properties in in?am-mation.It has been known that the inhibitions of cytokines result in anti-in?ammation(Denburg et al.,1991).Therefore,it was presumed that one of underlying mechanisms of anti-in?ammation by fangchinoline and tetrandrine is concerned with the inhibitory actions of cytokines which exhibit common biological properties in in?ammation.In addition,the further detailed studies are necessary for the inhibitory actions of cytokines by fangchi-noline and tetrandrine.
Meanwhile,calcium channel blockers have been demonstrated to have anti-in?ammatory action by inhibiting5-lipoxygenase which is present as an inactive form,and need calcium for activation (Salmon and Higgs,1987).Fangchinoline(Kim et al.,1997)and tetrandrine(King et al.,1988;Kim et al.,1997)have been reported as nonspeci?c calcium channel blockers.These results suggest the possibility that fangchinoline and tetrandrine might have anti-in?ammatory actions by inhibit-ing5-lipoxygenase.
Fangchinoline differs from tetrandrine in one of the side chains of one of its isoquinoline rings (OH substitution instead of OCH3).This minor difference in fangchinoline confers appreciable differences in inhibitory effects on cyclooxygenase and mIL-5activity from tetrandrine.All of the results taken together,it was presumed that OH of the isoquinoline rings of fangchinoline seems to be essential to exhibit inhibitory effects on cy-clooxygenase activity,and OCH3essential to ex-hibit inhibitory effects on mIL-5activity.But to inhibit hIl-6activity both OH and OCH3do not matter each other.
Moreover,this study offers valuable insights into structure-activity relationships of the bisben-zylisoquinolines.Studies on other natural and synthetic analogues of tetrandrine may indicate the importance of the parent benzylisoquinoline structure on immunopharmacological activity, and lead to the development of a new class of drugs.
4.Discussion
In this study,fangchinoline and tetrandrine showed anti-in?ammatory effects on mouse ear edema.These results are consistent with the stud-ies that fangchinoline and tetrandrine have anti-in?ammatory activities against formaldehyde-induced swelling on rat paws(Lu et al.,1957), and tetrandrine also have anti-in?ammatory ac-tivity in experimental burns in rabbits(Berezhin-skaya and Trutneva,1965).
In this experiment,cyclooxygenase activity was not inhibited by tetrandrine,but by fangchinoline. In consistent with the present study,it was re-ported that the action site of anti-in?ammation by tetrandrine is not on the cyclooxygenase enzyme complex,but on the phospholipase-mediated re-lease of arachidonic acid from the cell membrane (Teh et al.,1990),similar to the action of corticos-teroids(Di Rosa and Persica,1979). Additionally,tetrandrine showed potent in-hibitory effects on both of mIL-5and hIL-6,but fangchinoline only on hIL-6in this study.It had been demonstrated that production of IL-1by human monocytes was also inhibited by tetran-drine(Seow et al.,1989)and production of IL-1 and TNF-a was inhibited by fangchinoline(Onai
H.-S.Choi et al./Journal of Ethnopharmacology69(2000)173–179179
Acknowledgements
This study was supported by a research grant from the Korea Food and Drug Administration (1998).
References
Berezhinskaya,V.V.,Trutneva,E.A.,1965.Certain species of Stephania as sources for drugs.Izuch I Ispol’z Lekarstv Rastit Resursov SSSR(Leningrad:Med.)Sb.1964,320–324(Russia).
Denburg,J.A.,Gauldie,J.,Dolovich,J.,Ohtoshi,T.,Cox,G., Jordana,M.,1991.Structural cell-derived cytokines in allergic in?ammation.International Archives of Allergy and Applied Immunology.94(1-4),127–132.
Di Rosa,M.,Persica,P.,1979.Mechanism of inhibition of prostaglandin biosyntehsis by hydrocortisone in rat leuko-cytes.British Journal of Pharmacology.66,161–163. Huang,K.C.,1993.The Pharmacology of Chinese Herbs.CRC press,Florida,pp.118–120.
Kawase,T.,Ogata,S.,Origasa,M.,Burns, D.M.,1995.
1,25-dihydroxyvitamin D3promotes prostaglandin E1-in-duced differenciation of HL-60cells.Calci?ed Tissue Inter-national57(5),359–366.
Kim,H.S.,Zhang,Y.H.,Oh,K.W.,Ahn,H.Y.,1997.Vasodi-lating and hypotensive effects of fangchinoline and tetran-drine on the rat aorta and the stroke-prone spontaneously hypertensive rat.Journal of Ethnopharmacology58,117–123.
King,V.F.,Garcia,M.L.,Himmel,D.,Reuben,J.P.,Lam, Y.K.T.,Pan,J.X.,Han,G.Q.,Kaczorowski,G.J.,1988.
Interaction of tetrandrine with slowly inactivating calcium channels:characterization of calcium channel modulation by an alkaloid of chinese medicinal herb origin.Journal of Biological Chemistry.263,2238–2244.
Li,Q.,Xu,Y.,Zhon,Z.,Chen,Q.,Huang,Z.,Chen,S.,Zhan,
C.,1981.The therapeutic effect of tetrandrine on silicosis.
Chung Hua Chieh Ho Ho Hu Hsi Hsi Chi Ping Tsa Chih 4,321–328.
Lin,L.Z.,Shieh,H.L.,Angerhofer, C.K.,Pezzuto,J.M., Cordell,G.A.,Xue,L.,Johnson,M.E.,Ruangrungsi,N., 1993.Cytotoxic and antimalarial bisbenzylisoquinoline al-
kaloids from cyclea barbata.Journal of Natural Products 56,22–29.
Liu, B.,Zou, C.,Li,Y.,1983.Studies of the contents of glycosaminoglycans from lungs of silicotic rats and tetran-drine-treated silicotic rats.Ecotoxicology and Environmen-tal Safety7,323–329.
Lu,F.H.,Chang,T.M.,Fong,T.C.,1957.The antiphlogistic and anti-anaphylactic shock actions of tetrandrine and demethyl-tetrandrine.Acta Physiologica Sinica5(2),113–122.
Min,K.R.,Kim,Y.,Kang,S.H.,Mar,W.,Lee,K.S.,Ro,J.S., Lee,S.H.,1996.Natural Product Science2,56–74. Mossman,T.,1983.Rapid colorimetric assay for cellular growth and survival:application to proliferation and cyto-toxicity assays.Journal of Immunological Methods65, 55–63.
Nakamura,K.,Tsuchiya,S.,Sugimoto,Y.,Sugimura,Y., Yamada,Y.,1992.Histamine release inhibition activity of bisbenzylisoquinoline alkaloids.Planta Medica58(6),505–508.
Onai,N.,Tsunokawa,Y.,Suda,M.,Watanabe,N.,Nakamura, K.,Sugimoto,Y.,Kobayashi,Y.,1995.Inhibitory effects of bisbenzylisoquinoline alkaloids on induction of proin?am-matory cytokines,interleukin-1and tumor necrosis factor alpha.Planta Medica61(6),497–501.
Salmon,J.A.,Higgs,G.A.,1987.Prostaglandins and leukotrienes as in?ammatory mediators.British Medical Bulletin43,285–296.
Seow,W.K.,Ferrante,A.,Li,S.Y.,Thong,Y.H.,1989.Sup-pression of human monocyte interleukin1production by the plant alkaloid tetrandrine.Clinical and Experimental Immunology75,47–51.
Teh,B.S.,Seow,W.K.,Li,S.Y.,Thong,Y.H.,1990.Inhibition of prostagladin and leukotriene generation by the plant alkaloids tetrandrine and berbamine.International Journal of Immunopharmacology12(3),321–326.
Tonneli,G.,Thiabault,L.,Ringler,I.,1965.A bioassay for the concomitant assessment of the antiphlogistic and thymolytic activities of topically applied corticoids.Endocrinology77, 625–634.
Tsai,J.J.,Joseph,K.H.M.,Wang,T.F.,Wang,S.R.,Kao,
D.H.,1995.The modulatory effect of tetrandrine on the
CD23,CD25and HLA-DR expression and cytokine pro-duction in different groups of asthmatic patients.Interna-tional Archives of Allergy and Immunology108,183. Yamaguchi,K.,1970.Spectra Data of Natural Products1,559.
.
